activities involving genetically modified animals risk assessment this form is should be completed with reference to the scientifi
Activities Involving Genetically Modified Animals
Risk Assessment
This form is should be completed with reference to the Scientific
Advisory Committee on Genetic Modification (SACGM) Compendium of
Guidance Parts 1 and 5 which can be accessed either via the URL
address: http://www.hse.gov.uk/biosafety/gmo/acgm/acgmcomp/ and
available on SPIDER under the safety link and GM tab.
SACGM Compendium of Guidance – Brief Summary
GM animals are those where their genetic material has been altered
using a method that does not occur naturally, but excluding chemical
or physical mutagenesis. In its broadest sense this includes both
vertebrates and invertebrates.
You will need to take account of the specific species on a case by
case basis and the legislation covers the breeding on of a genetically
modified animal. Even when a GM animal is crossed with a non-GM animal
the progeny will be considered to be GMOs.
All activities involving GM animals must be risk assessed in relation
to human health and safety as well as the environment and records
require to be kept for 10 years.
In outline, the recommended procedure is:
i.
risk assessment for environmental protection (including hazard
identification, assessment of likelihood and consequences;
determination of risk; assignment of risk management measures to
protect the environment);
ii.
risk assessment for human health and safety;
iii.
assignment of the final containment and control requirements
(adjusting (i) to take account of (ii)).
As part of the consideration under (ii) it is important to determine
whether the GM animal is more likely to cause harm to humans than the
non-modified parental organism. This is so you can determine whether
the activity has to be notified.
The hazards that need to be considered will usually be:
(i) capacity to survive, establish, disseminate, compete with and / or
displace other animals;
(ii) (direct) adverse effects on animals and plants;
(iii) potential for transfer of genetic material between the GMO and
other organisms;
(iv) products of gene expression, particularly if they are toxic or
otherwise biologically active;
(v) phenotypic and genetic stability; and
(vi) other negative effects on organisms.
The Genetically Modified Organisms (Contained Use) Regulations 2014 do
not set out detailed containment levels for GM animals. However, the
SACGM guidance takes the approach that a basic level of containment
should be applied to all GM animals. It is recognised that for the
majority of GM animals currently being used the modified animal poses
no additional risk to workers over those that would be expected from
the parental (unmodified) animal. These intrinsic hazards will include
such things as scratches, bites, allergenicity and zoonoses
(especially the possibility of persistent or latent infection).
All of these aspects must be considered in any risk assessment so that
appropriate containment and control measures can be assigned. However,
these potential hazards are, of course, common to all animals, whether
GM or not. The final containment and control measures assigned must be
sufficient to reduce all risks to both humans and the environment to
“low” or “effectively zero” and prevent harm from occurring is termed
’not notifiable’. Examples of the types of GM animals for which this
containment is appropriate are likely to include “knockout” mice.
By analogy, hazards resulting from the genetic modification which
could give risk to “medium” or even “severe” consequences for human
health the risk would be truly greater than that posed by the
non-modified animal is termed ’notifiable’ where a series of
additional measures to control specific risks is required and would
trigger a notification to the HSE via the University GM Safety
Committee (GMSC).
The risk assessment should consider the modified organism, the nature
of the activity, or process, and the nature of any product. In some
cases the level of containment and control may be dictated by the risk
posed by the product or process rather than by the GMO itself.
Where it is proposed to inoculate animals with viable GMMs, animal
containment corresponding to that used in the laboratory for the
micro-organisms concerned, modified by the requirements specific to
animal houses, should be used (see Part 3E of the Compendium of
Guidance).
Using the following guidelines you will need to assess whether your GM
animal requires notification to HSE or not.
Notification to HSE is NOT required for GM animals which exhibit any
of the following traits or properties:
*
they are incapable of surviving in the environment in the UK; or,
*
they have limited ability to transfer genetic material to UK
animal species; or,
*
the genetic modification does not increase the risk to human
health or the environment above that of the non-modified parental
organism;
*
and the GM animals have either not been inoculated or have been
inoculated with Class 1 GM material or other pathogens
Notification to HSE IS required for GM animals which have any of the
following characteristics:
*
the animals could cause harm to humans or the environment if they
escaped from the containment facility, and they have the ability
to transfer novel genetic material to UK animal species; or,
*
the animals could establish outside the facility; or,
*
the GM increases the level of risk to human health or the
environment above that of the non-modified parental organism;
*
or the animals have been inoculated with a GM material or other
pathogen that is above Class 1
Is notification to HSE required? NO YES
If ’YES’ you will be required to complete a separate risk assessment
for the University Genetic Modification Safety Committee which has to
be approved by the Health and Safety Executive.
Principal Investigator:
Proposer:
Signature of Proposer:
Date:
Risk Assessment for Containment of an Individual Strain of Genetically
Modified Animal (GMO).
1. SPECIES, GM NAME & STRAIN/BACKGROUND OF GM ANIMAL:
2. DESCRIPTION OF THE GENETIC MODIFICATION (to include: The origin and
activity of the inserted material, and, where known, levels of
expression, the location of the material on plasmids or on
chromosomes. Key references, where appropriate and where known, should
be included)
3. ASSESSMENT OF THE HAZARDS POSED TO HUMAN HEALTH BY THE GMO IN
COMPARISON TO THE NON-MODIFIED HOST (e.g. Increased allergenic or
toxic effects, acting as a human vector, adverse effects to humans
from altered behaviour or physical factors)
4. ASSESSMENT OF THE HAZARDS POSED TO THE ENVIRONMENT BY THE GMO IN
COMPARISON TO THE NON-MODIFIED HOST (e.g. Does the modification alter
the capacity of the organism to survive?)
5. ASSESSMENT OF THE CONSEQUENCES OF THE GMO ESCAPING FROM CONTAINMENT
IN TERMS OF RISK TO THE HEALTH OF THE HUMAN POPULATION AND TO THE
ENVIRONMENT (To include the potential of transfer of genetic material
to other populations)
 EUROPEAN TREATY SERIES NO 166 EUROPEAN CONVENTION ON
EUROPEAN TREATY SERIES NO 166 EUROPEAN CONVENTION ON LE PROJET D’INSCRIPTION DE LA FORÊT BORÉALE PIMACHIOWIN AKI
LE PROJET D’INSCRIPTION DE LA FORÊT BORÉALE PIMACHIOWIN AKI FILOSOFIA DE PLATÓ 1 LA REALITAT EN PLATÓ DUALISME
FILOSOFIA DE PLATÓ 1 LA REALITAT EN PLATÓ DUALISME VEHICLES OF VICTORY LLC PO BOX 874 WINSTON GA
VEHICLES OF VICTORY LLC PO BOX 874 WINSTON GA WAS IST EIN KLIMADIAGRAMM? KLIMA IST EINE BESCHREIBUNG DES
WAS IST EIN KLIMADIAGRAMM? KLIMA IST EINE BESCHREIBUNG DES SOPHIE IS ELEVEN YEARS OLD AND SHE LIVES IN
SOPHIE IS ELEVEN YEARS OLD AND SHE LIVES IN THE IONIC STYLE IONIC EVOLVED IN IONIA ON THE
THE IONIC STYLE IONIC EVOLVED IN IONIA ON THE BIHARI SZABINA PUJARICA SEJ AKI UTÁLJA A KÖRLEVELEKET ÉS
BIHARI SZABINA PUJARICA SEJ AKI UTÁLJA A KÖRLEVELEKET ÉS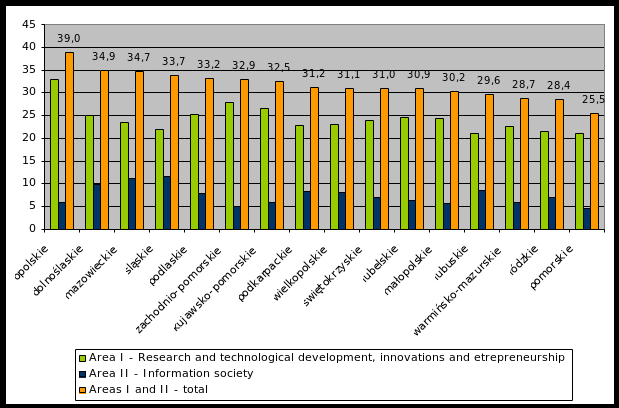 METROPOLIES AND BIG CITIES AS REGIONS’ GROWTH LOCOMOTIVES ANALYSES
METROPOLIES AND BIG CITIES AS REGIONS’ GROWTH LOCOMOTIVES ANALYSES POWERPLUSWATERMARKOBJECT357922611 SUPPLEMENTARY TERMS FROM THE CLAUSE BANK 1 ORGANISATION
POWERPLUSWATERMARKOBJECT357922611 SUPPLEMENTARY TERMS FROM THE CLAUSE BANK 1 ORGANISATION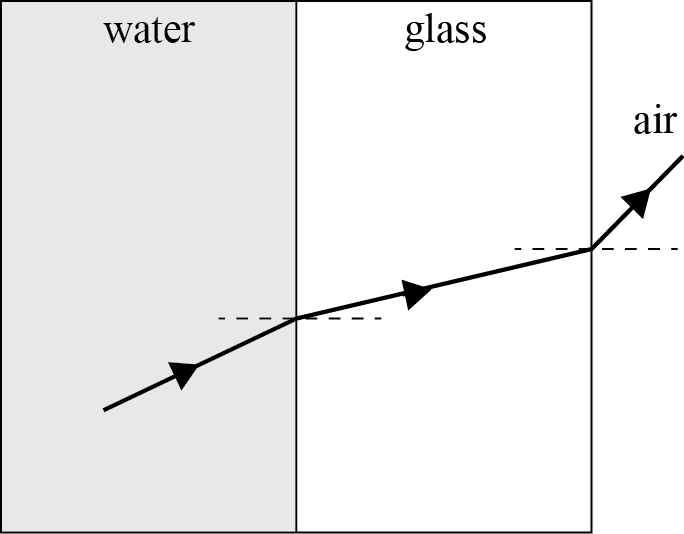 NCEA LEVEL 1 PHYSICS (90938) 2011 — PAGE 5
NCEA LEVEL 1 PHYSICS (90938) 2011 — PAGE 5 GOATSTOWN ROAD CYCLE ROUTE FREQUENTLY ASKED QUESTIONS FREQUENTLY ASKED
GOATSTOWN ROAD CYCLE ROUTE FREQUENTLY ASKED QUESTIONS FREQUENTLY ASKED