institutional review board (irb) guidelines for scientific pre-review 1. introduction investigator-initiated research is frequen
Institutional Review Board (IRB)
GUIDELINES FOR SCIENTIFIC PRE-REVIEW
1. Introduction
Investigator-initiated research is frequently the most creative and
cutting-edge type of human subjects research. However, this type of
research does not always undergo scientific peer review before
submission to the IRB, particularly if it is not sponsored (i.e.,
funded). Therefore, it is the policy of the IRB that unsponsored,
non-exempt, investigator-initiated research protocols must be
submitted to a departmental or divisional scientific review
committee/representative before submission to the IRB. The scientific
reviewer(s) should have the appropriate expertise to evaluate the
scientific merit of the research protocol. The goal of this policy is
to assure that the conduct of research conforms to the highest
standards of research methodology, while most effectively minimizing
risks to volunteer subjects. Finally, consistent with the Institute of
Medicine’s 2002 Report: “Responsible Research: A Systems Approach to
Protecting Research Participants,” this policy is designed to allow
the IRB to focus its efforts primarily on ethics issues regarding
human subjects protections. While the IRB will continue to review
issues of scientific design and subject safety as needed, that role
will be a secondary one; the scientific review policy will enable
those with the most relevant scientific expertise to have principal
responsibility for assessing scientific merit.
2. Studies Subject to Scientific Review Requirement
Prior to submission to the IRB, protocols for investigator-initiated,
unsponsored, non-exempt research must undergo review for scientific
merit, to include the following elements: background literature
review, scientific design, statistical data analysis, and research
subject risk assessment.
Examples of investigator-initiated research that are not subject to
the scientific review requirement include research supported by:
*
Federal agencies such as NIH or USPHS
*
National or local funding agencies such as American Heart
Association, American Cancer Society, Alzheimer’s Association,
etc.
Research protocols that qualify for exemption from federal regulations
per 45 CFR 46.101(b) also do not require scientific review.
3. Scientific Review Instructions
Before submission to the IRB, unsponsored, non-exempt,
investigator-initiated research protocols must be submitted to a
departmental or divisional Scientific Review committee or
representative. Reviewer(s) should have appropriate expertise to
evaluate the scientific merit of the protocol. The
committee/representative will review the protocol for adequacy of
background literature review, scientific design, data analysis, and
risk/safety oversight. Reviewer(s) are asked to provide an opinion as
to whether risks to participants are minimized by using procedures
consistent with sound research design that do not unnecessarily expose
participants to risk AND whether risks to participants are reasonable
in relation to anticipated benefits to participants and the importance
of the knowledge that may reasonably be expected to result.
Scientific Reviewers are encouraged to use the Scientific Review
Worksheet to aid them in this review. The completed form need not be
submitted to the IRB, but can be retained by the reviewer or kept in
the PI’s research records.
In some departments, the committee that performs this scientific
review role is called a Protocol Preparation Committee (PPC). Other
departments may provide scientific review in a less structured manner.
For a known list of unit-specific scientific review requirements,
please see the Department/Division-Specific Guidelines for eIRB
Submission Pre-Review Requirements.
Committee members/representatives conducting the scientific review
cannot be listed as research team members on the protocol being
reviewed. Scientific reviewers should be in a position to conduct an
objective review; therefore, it is IRB policy to not allow scientific
reviews to be completed by a research team member. The review also may
not be done by the Department Chairperson/Faculty Advisor.
Once the departmental or divisional committee/representative has
completed the scientific review, this person can pre-approve the
protocol in the eIRB system (for electronic protocols) or sign the
attached Scientific Review Form (which will get uploaded as an
attachment to the IRB Application in eIRB). This will confirm that the
protocol has been scientifically reviewed.
The research protocols and related documents will then undergo
standard review by the IRB. All protocols that are deferred by the IRB
for lack of scientific merit or lack of risk/safety monitoring will be
returned to the investigator. These deferred protocols may be required
to have a new scientific review prior to re-submission to the IRB.
Institutional Review Board (IRB)
Scientific Pre-Review Form
This form is to be submitted to the Institutional Review Board (IRB)
office as an attachment to unsponsored, non-exempt,
investigator-initiated research protocols. Scientific review should be
completed within the eIRB system unless otherwise directed to upload
this completed form as an attachment to the IRB Application in eIRB.
Date:
Department/Division:
Principal Investigator:
Study Title:
The above referenced human research protocol was reviewed thoroughly
and critically for adequacy of background literature review,
scientific design, data analysis, and risk/safety oversight. In
signing here, I attest that it is my opinion that in this research
study, as designed or with changes I’ve requested be made, risks to
participants are minimized by using procedures consistent with sound
research design that do not unnecessarily expose participants to risk
AND risks to participants are reasonable in relation to anticipated
benefits to participants and the importance of the knowledge that may
reasonably be expected to result.
Printed Name
Signature
Title
Department/Division
Note: at least one scientific reviewer is required. The reviewer(s)
listed in the table above may not be a member of the research team or
the Department Chair/Faculty Advisor.
3
Investigator Initiated Policy effective 12/1/03, Revised 07/2019
 MECHATRONIKAI MÉRNÖKI MESTERSZAK 2NMM0 INTELLIGENS BEÁGYAZOTT MECHATRONIKAI RENDSZEREK SZAKIRÁNY
MECHATRONIKAI MÉRNÖKI MESTERSZAK 2NMM0 INTELLIGENS BEÁGYAZOTT MECHATRONIKAI RENDSZEREK SZAKIRÁNY COLEGIO SANTA MARTA “LITERATURA E IDENTIDAD” PLAN DIFERENCIADO LENGUAJE
COLEGIO SANTA MARTA “LITERATURA E IDENTIDAD” PLAN DIFERENCIADO LENGUAJE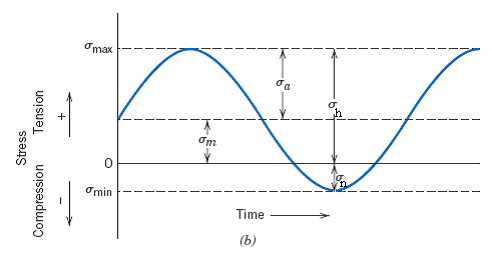 ÚNAVOVÝ LOM ÚNAVA MATERIÁLU ÚNAVA KONŠTRUKCNÝCH MATERIÁLOV JE DEGRADACNÝ
ÚNAVOVÝ LOM ÚNAVA MATERIÁLU ÚNAVA KONŠTRUKCNÝCH MATERIÁLOV JE DEGRADACNÝ SR EK3 – TEKLIF DOSYASI SATIN ALMA REHBERI TEKLİF
SR EK3 – TEKLIF DOSYASI SATIN ALMA REHBERI TEKLİF EXTERNAL AND INTERNAL PRESSURES ON THE TRANSLATOR RELATIONSHIP TO
EXTERNAL AND INTERNAL PRESSURES ON THE TRANSLATOR RELATIONSHIP TO ABCONCURSOSCOM CFRAY JUAN GIL 5 ALCALA DE HENARES MADRID
ABCONCURSOSCOM CFRAY JUAN GIL 5 ALCALA DE HENARES MADRID WORK PLACEMENT EVALUATION NAME LOCATION OF PLACEMENT DATES
WORK PLACEMENT EVALUATION NAME LOCATION OF PLACEMENT DATES ECETRADECWP7GE620076 PAGE 7 E UNITED NATIONS ECONOMIC
ECETRADECWP7GE620076 PAGE 7 E UNITED NATIONS ECONOMIC APPLICATION FOR PUTTING HIGHER DEGREES STUDY IN ABEYANCE TO
APPLICATION FOR PUTTING HIGHER DEGREES STUDY IN ABEYANCE TO KULTUR NES KOMMUNE SØKNAD OM DRIFTSTILSKUDD FOR IDRETTSORGANISASJONER
KULTUR NES KOMMUNE SØKNAD OM DRIFTSTILSKUDD FOR IDRETTSORGANISASJONER  SCHEMA RELEASE 26 SCHEMA RELEASE ASEXML SCHEMA WORKING GROUP
SCHEMA RELEASE 26 SCHEMA RELEASE ASEXML SCHEMA WORKING GROUP BOLETÍN DE INSCRIPCIÓN () DDª NIF DOMICILIO TFNO CIUDAD
BOLETÍN DE INSCRIPCIÓN () DDª NIF DOMICILIO TFNO CIUDAD