checkpoint task =============== reactivity of metals ==================== instructions and answers for teachers --------
Checkpoint Task
===============
Reactivity of Metals
====================
Instructions and answers for teachers
-------------------------------------
These instructions cover the student activity section which can be
found on page 5. This Checkpoint Task should be used in conjunction
with the KS3-4 GCSE (9-1) Twenty First Century Science Chemistry B
Transition Guide: Reactivity of Metals, which supports OCR GCSE (9-1)
Twenty First Century Science Chemistry B.
When distributing the activity section to the learners either as a
printed copy or as a Word file you will need to remove the teacher
instructions section.
Introduction
The checkpoint task is called the ‘reactivity challenge’. There are
three mini activities that become progressively more challenging. The
task is designed to initially assess learners’ understanding of the
properties of metals in challenge 1, progressing to displacement in
challenge 2 and finally writing balanced equations in challenge 3. The
organisation of the activity will depend on the nature of the group.
With motivated high ability, or competitive groups it would be ideal
to turn each challenge as a race. Less confident or lower ability
groups may prefer to work through it at their own pace. However, as
the activity covers a range of skills it would be beneficial to check
answers after each challenge. Problems and barriers with the
individual challenges can be resolved challenge by challenge. These
tasks will enable the class teacher to assess what knowledge and
skills can be recalled from the Key Stage 3 topic before moving into
the GCSE content. Answers are provided in the teacher’s section.
Teacher preparation
To run this activity you could either split your group into small
groups or ask them to work individually. You may wish to supply
learners with periodic tables, or you may have chosen to watch some of
the videos suggested in the transition resources to refresh learners’
knowledge.
Challenge 1 – Properties of metals
Complete the table using your knowledge of metals. The first one has
been done for you.
Metal
Property
Use
Copper
Conducts electricity
Electrical wires
Aluminium
Conducts heat
Sauce pans
Gold
Malleable, unreactive
Jewellery
Iron
Strong
Bridges
Challenge 2 – Displacement
You could ask the learners to complete this experiment instead of
giving the results.
Some learners did an experiment and observed reactions with the
following metals and their metal ion solutions. They placed ticks and
crosses when they saw a reaction take place.
Copper sulphate
Magnesium sulphate
Zinc
sulphate
Aluminium sulphate
Copper
x
x
x
x
Magnesium
x
Zinc
x
x
x
Aluminium
x
x
Write a word equation for each of the reaction where there is a tick.
Magnesium + copper sulfate magnesium sulfate + copper
Magnesium + zinc sulfate magnesium sulfate + zinc
Magnesium + aluminium sulfate magnesium sulfate + aluminium
Zinc + copper sulfate Zinc sulfate + copper
Aluminium + copper sulfate aluminium sulfate + copper
Aluminium + zinc sulfate aluminium sulfate + zinc
Challenge 3 – Reactivity equations
Complete the word equations and then write balanced equations for the
reactions.
Copper + hydrochloric acid copper chloride + hydrogen
Cu + 2HCl CuCl2 + H2
Copper + sulfuric acid copper sulfate + hydrogen
Cu + H2SO4 CuSO4 + H2
Copper + nitric acid copper nitrate + hydrogen
Cu + 2HNO3 Cu(NO3)2 + H2
Magnesium + hydrochloric acid magnesium chloride + hydrogen
Mg + 2HCl MgCl2 + H2
Magnesium + sulfuric acid magnesium sulfate + hydrogen
Mg + H2SO4 MgSO4 + H2
Magnesium + nitric acid magnesium nitrate + hydrogen
Mg + 2HNO3 Mg(NO3)2 + H2
Zinc + hydrochloric acid zinc chloride + hydrogen
Zn + 2HCl ZnCl2 + H2
Zinc + sulfuric acid zinc sulfate + hydrogen
Zn + H2SO4 ZnSO4 + H2
Zinc + nitric acid zinc nitrate + hydrogen
Zn + 2HNO3 Zn(NO3)2 + H2
We’d like to know your view on the resources we produce. By clicking
on ‘Like’ or ‘Dislike’ you can help us to ensure that our resources
work for you. When the email template pops up please add additional
comments if you wish and then just click ‘Send’. Thank you.
If you do not currently offer this OCR qualification but would like to
do so, please complete the Expression of Interest Form which can be
found here: www.ocr.org.uk/expression-of-interest
Checkpoint Task
===============
Reactivity of Metals
====================
Learner Activity
----------------
Introduction
These three challenges are to check your understanding from Key Stage
3.
Challenge 1 – Properties of metals
Complete the table using your knowledge of metals. The first one has
been done for you.
Metal
Property
Use
Copper
Conducts electricity
Electrical wires
Sauce pans
Jewellery
Bridges
Challenge 2 – Displacement
Some learners did an experiment and observed reactions with the
following metals and their metal ion solutions. They placed ticks and
crosses when they saw a reaction take place.
Copper sulphate
Magnesium sulphate
Zinc
sulphate
Aluminium sulphate
Copper
x
x
x
x
Magnesium
x
Zinc
x
x
x
aluminium
x
x
Write a word equation for each of the reaction where there is a tick.
The first one has been done for you.
Magnesium + copper sulfate magnesium sulfate + copper
Extension task
Challenge 3 – Reactivity equations
Complete the word equations and then write balanced equations for the
reactions.
C opper + hydrochloric acid copper chloride + hydrogen
Cu + 2HCl CuCl2 + H2
C opper + sulfuric acid
C opper + nitric acid
M agnesium + hydrochloric acid
M agnesium + sulfuric acid
M agnesium + nitric acid
Zinc + hydrochloric acid
Z inc + sulfuric acid
Z inc + nitric acid
Copyright © OCR 2016
Version 1
 GRADE 7 MODULE 1 UNIT 2 LESSON 9 MIDUNIT
GRADE 7 MODULE 1 UNIT 2 LESSON 9 MIDUNIT GABRIEL WWWCEMAEDUAR~GEC02C7DOC MAVIE 9001100 23 DE ABRIL DE 2004
GABRIEL WWWCEMAEDUAR~GEC02C7DOC MAVIE 9001100 23 DE ABRIL DE 2004 FACILITY RENTAL INFORMATION T HE GAITHER FAMILY AUDITORIUM
FACILITY RENTAL INFORMATION T HE GAITHER FAMILY AUDITORIUM  DIRECTION DEPARTEMENTALE DE LA COHESION SOCIALE MEMOIRE DE PROPOSITION
DIRECTION DEPARTEMENTALE DE LA COHESION SOCIALE MEMOIRE DE PROPOSITION CUESTIONARIO PARA ACADÉMICOS FECHA DE APLICACIÓN DE LA ENCUESTA
CUESTIONARIO PARA ACADÉMICOS FECHA DE APLICACIÓN DE LA ENCUESTA FD III SPANISCH SS 2008 – MARCHAND MIKULIĆ
FD III SPANISCH SS 2008 – MARCHAND MIKULIĆ 17072018 LA FUNDACIÓN SAGASTA ORGANIZA LA IX EDICIÓN DE
17072018 LA FUNDACIÓN SAGASTA ORGANIZA LA IX EDICIÓN DE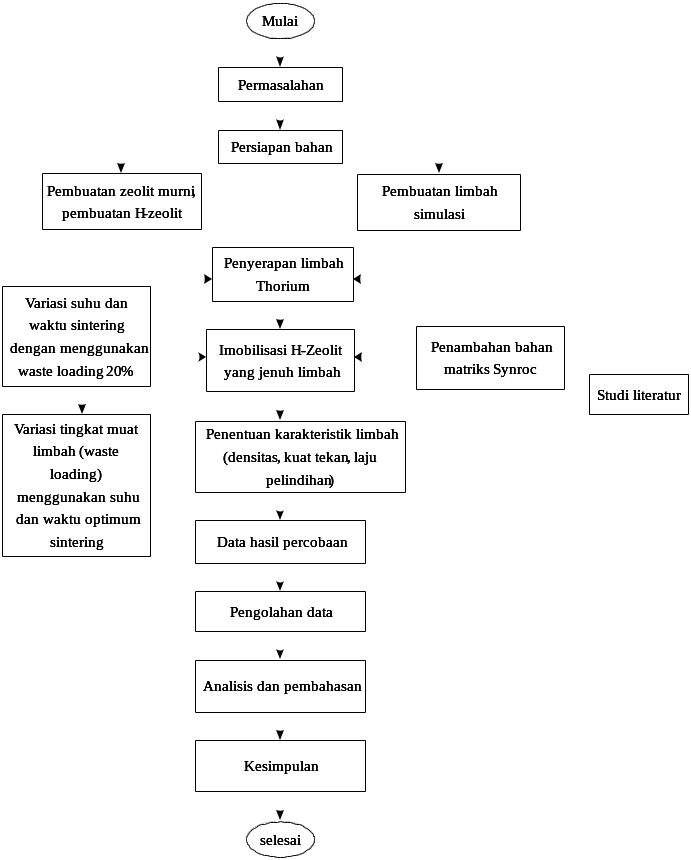 IMOBILISASI LIMBAH RADIOAKTIF MENGANDUNG THORIUM MENGGUNAKAN BAHAN MATRIKS SYNROC
IMOBILISASI LIMBAH RADIOAKTIF MENGANDUNG THORIUM MENGGUNAKAN BAHAN MATRIKS SYNROC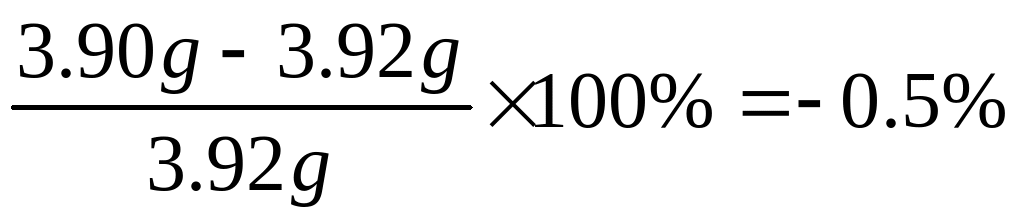 ACCURACY AND PRECISION WORKSHEET ANSWER KEY QUESTIONS 26 2)
ACCURACY AND PRECISION WORKSHEET ANSWER KEY QUESTIONS 26 2) TECHNICAL JOB FAMILY (TECHNICAL GRADES 1 – 6) JUNE
TECHNICAL JOB FAMILY (TECHNICAL GRADES 1 – 6) JUNE SPORTSCHÜTZENVERBAND ALFELD FEIERT SEINE MAJESTÄTEN ZUM KÖNIGSBALL DES SPORTSSCHÜTZENVERBANDES
SPORTSCHÜTZENVERBAND ALFELD FEIERT SEINE MAJESTÄTEN ZUM KÖNIGSBALL DES SPORTSSCHÜTZENVERBANDES FORMATO F – 3 PROPUESTA ECONÓMICA MEDICAMENTO CASA COMERCIAL
FORMATO F – 3 PROPUESTA ECONÓMICA MEDICAMENTO CASA COMERCIAL