pct/mia/v/2 page 5 wipo pct/mia/v/2 original: english date: november 25, 1994 world intellectual property organizat
PCT/MIA/V/2
page 5
WIPO
PCT/MIA/V/2
ORIGINAL: English
DATE: November 25, 1994
WORLD INTELLECTUAL PROPERTY ORGANIZATION
GENEVA
International patent cooperation union
(PCT union)
Meeting of international authorities
under the PCT
Fifth Session
Geneva, November 28 to December 1, 1994
THE TRILATERAL PATENT-EJU-SEQUENCE DATABASE:
POSSIBILITIES TO USE THAT DATABASE BY
DESIGNATED AND/OR ELECTED OFFICES
prepared by the European Patent Office
The Trilateral Patent–
EJU–Sequence Database : possibilities to use
that Database by
designated and/or Elected Offices
The present document is intended to inform the representatives
of the international authorities not being parties to the EPO–
JPO-USPTO Cooperation of the ongoing activities relating to the
Trilateral Patent Sequence Database.
I. The Trilateral Patent Sequence Database
1. In the framework of the Trilateral Cooperation, the three Offices
have decided to capture all nucleotide and amino acid sequences from
all published patent documents. In order to capture these sequence
data, each Office is collaborating with a contractor. The JPO is
collaborating
with JAPIO, the USPTO with the NCBI (National Center for Biotechnology
Information) and the EPO with the EMBL Data Library in Heidelberg,
Germany (to be changed in the European Bioinformatics Institute,
Hinxton, United
Kingdon in September 1994).
2. The data to be captured comprise two parts, a backfile and a
frontfile. The backfile consists of sequence- containing patent
documents since 1960 for which the sequences are not available in
electronic form. The frontfile consists of sequence-containing
documents (published after August 1, 1993) for which the sequence
information has been provided by the applicant in electronic form.
3. For the backf ile the sequences are selected by
annotators, entered twice by data entry staff, merged
with the patent document frontfile information and added to the
database. For the frontpage information
(applicant, publication date etc.), the sequences are,
after publication of the patent document, merged with the frontpage
information of the patent document and added to the database. It is
estimated that the EPO part of the backfile will be completed by the
end of this year. The capture of the frontfile data will start
shortly.
4. Each office is capturing the sequences from those patent documents
for which that office has acted as priority country. For first filings
in the EPO Contracting States,
the EPO captures, in principle, the second EP and PCT applications and
for those first filings not leading to a
EP or PCT application, it captures on the basis of the national
applications which are watched for that purpose. This in addition to
EP-applications being first filings.
The three parts of the database so created are exchanged
by the three offices and an entire database is generated which is made
available to all interested parties at
marginal costs.
5. The database will be made available via the existing
media provided by, amongst others, the EMBL Data Library and will
therefore be searchable with the commonly used search tools.
II. Possibility to use the Trilateral Patent Sequence Database by the
Designated, and/or Elected Offices
…/…
6. In order to avoid that Designated and/or Elected Offices
have to ask the applicants for a sequence listing in
computer-readable form it is proposed that these offices
make use of the Trilateral Patent Sequence Database in
case they would like to access the sequences from a given
sequence listing. The following aspects should be
considered:
a) Do the Elected Offices and/or the Designated Offices
need access to the computer-readable form of the
sequence listing as filed or do they need access to
the searchable sequences of the sequence listing?
b) How are the Elected and/or Designated Offices going
to access the database?
7. In order to have access to the Trilateral Patent Sequence
Database, the designated/elected Office would need to
have either an on-line connection to an appropriate host
(e.g. European Patent Office) or a dedicated computer
system equipped with the necessary software and
databases .
8. If a designated or elected Office wishes to carry out a
supplementary search (e.g. the EPO, where the
international search has been carried out by the USPTO or
the JPO) , that designated or elected Office may access
the database as described under 7 on the basis of the WO
publication number and the hardcopy of the sequence
listing.
The proposed procedure implies that the sequence from all
WO documents are present in the database.
9. An example of a database entry created from a WO document
is included (see Annex).
10. The question of costs for accessibility of the
designated/elected Offices to the Trilateral Patent
Sequence Listing Database is not addressed in the present
document.
[Annex follows]
AC
A00l42;
XX
DT
11-FEB-1993 (Rel. 34, Created)
DT
11-FEB-1993 (Rel. 34, Last updated, Version 1)
XX
DE
H. sapiens LAG–2 gene encoding lymphokine LAG–2
XX
KW
LAG–2 gene; lymphokine.
XX
OS
Homo sapiens (human)
OC
Eukaryota; Animalia; Metazoa; Chordata; Vertebrata; Mammalia;
OC
Theria; Eutheria; Primates; Haplorhini; Catarrhini; Hominidae.
XX
PN
WO9003394–A/1
PD
05-APR-1990
PF
26-SEP-1989 WO89FR00491
PR
26-SEP-1988 FR880012538
PA
Roussel UCLAF.
PI
Hercend T.;
PT
"NEW LIMPHOKINES, DMA SEQUENCES CODING FOR SAID LIMPHOKINES
PT
AND PHARMACEUTICAL COMPOSITIONS CONTAINING SAID LIMPHOKINES";
PC
;
XX
FH
Key
Location/Qualifiers
FH
FT
source
1..634
FT
FT
/organism="Homo sapiens"
FT
CDS
25..462
FT
/gene="LAG–2"
FT
/product= "lympholine LAG-2"
XX
SQ
Sequence 634 BP; 131 A; 203 C; 169 G; 131 T; 0 other;
cggcatctca
gcggctgccc
caccatggct
acctgggccc
tcctgctcct
tgcagccatg
60
ctcctgggca
acccaggtct
ggtcttctct
cgtetgagcc
ctgagtacta
cgacctggca
120
agagcccacc
tgcgtgatga
ggagaaatcc
tgcccgtgcc
tggcccagga
gggcccccag
180
ggtgacctgt
tgaccaaaac
acaggagctg
ggccgtgact
acaggacctg
tctgacgata
240
gtccaaaaac
tgaagaagat
ggtggataag
cccacccaga
gaagtgtttc
caatgctgcg
300
acccgggtgt
gtaggacggg
gaggtcacga
tggcgcgacg
tctgcagaaa
tttcatgagg
340
aggtatcagt
ctagagttat
ccagggcctc
gtggccggag
aaactgccca
gcagatctgt
420
gaggacctca
ggttgtgtat
accttctaca
ggtcccctct
gagccctctc
accttgtcct
480
gtggaagaag
cacaggctcc
tgtcctcaga
tcccgggaac
gtcagcaacc
tctgccggct
540
cctcgcttcc
tcgatccaga
atccactctc
cagtctccct
cccctgactc
cctctgctgt
600
cctcccctct
caggagaata
aagtgtcaag
caag
634
//
ID
A00144
standard; DNA; PRI; 705 BP.
XX
A00144;
AC
XX
DT
11-FEB-1993 (Rel. 34, Created)
DT
11-FEB-1993 (Rel. 34, Last updated, Version 1)
XX
DE
H. sapiens LAG-2 gene promoter region
XX
KW
XX
OS
Homo sapiens (human)
OC
Eukaryota; Animalia; Metazoa; Chordata; Vertebrata; Mammalia;
OC
Theria; Eutheria; Primates; Haplorhini; Catarrhini; Eominidae.
XX
PN
WO9003394-A/3
[End of Annex and of document]
 TEMA 40 VISADO DE MEDICAMENTOS III VISADO DE OTROS
TEMA 40 VISADO DE MEDICAMENTOS III VISADO DE OTROS ABSENCE DE PROCÈSVERBAUX DU COMITÉ MÉDICAL DÉPARTEMENTAL DU MORBIHAN
ABSENCE DE PROCÈSVERBAUX DU COMITÉ MÉDICAL DÉPARTEMENTAL DU MORBIHAN TENDER DOCUMENT FOR 40 MBPS INTERNET CONNECTION THROUGH RADIO
TENDER DOCUMENT FOR 40 MBPS INTERNET CONNECTION THROUGH RADIO ATTACHMENT 4 COMMUNITY SERVICES CLIENT INFORMATION FORM NOTE IF
ATTACHMENT 4 COMMUNITY SERVICES CLIENT INFORMATION FORM NOTE IF PÁGINA | 3 XIV JORNADAS DE CONVIVENCIA PADRES –
PÁGINA | 3 XIV JORNADAS DE CONVIVENCIA PADRES – SEMINAR NASIONAL MEMBANGUN AGRIBISNIS UNTUK MENGGERAKKAN PEREKONOMIAN NASIONAL YANG
SEMINAR NASIONAL MEMBANGUN AGRIBISNIS UNTUK MENGGERAKKAN PEREKONOMIAN NASIONAL YANG MINISTERIO DE ENERGIA Y MINAS PROYECTO ELIMINACIÓN DE PASIVOS
MINISTERIO DE ENERGIA Y MINAS PROYECTO ELIMINACIÓN DE PASIVOS ŻYCIE JEST BEZCENNYM DAREM… 17 MAJA – MAJÓWKA DLA
ŻYCIE JEST BEZCENNYM DAREM… 17 MAJA – MAJÓWKA DLA TIME4ME OCTOBER 2016 TO SEPTEMBER 2017 WHAT IS A
TIME4ME OCTOBER 2016 TO SEPTEMBER 2017 WHAT IS A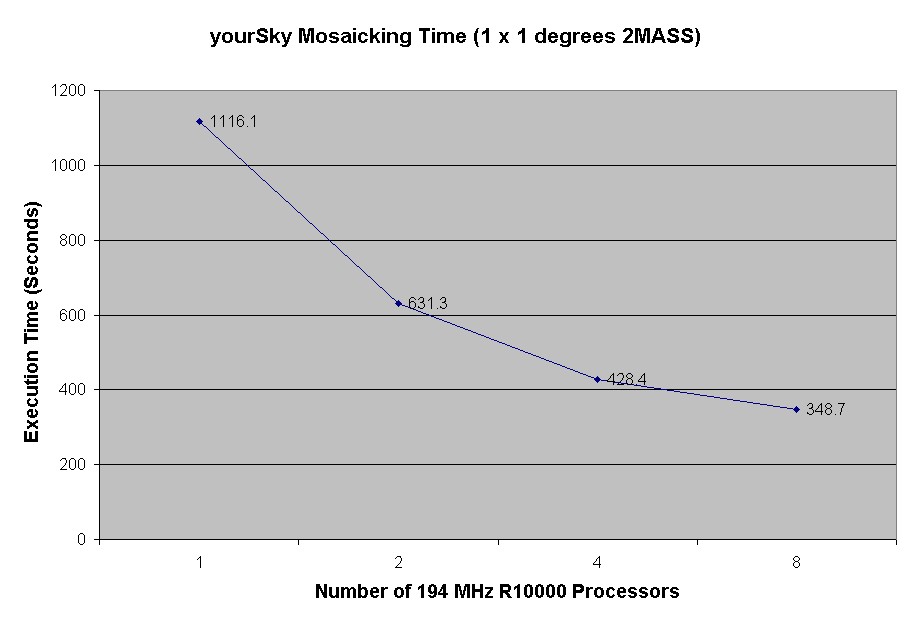 BASELINE PERFORMANCE YOURSKY MOSAICKING CODE CODE DESCRIPTION THE
BASELINE PERFORMANCE YOURSKY MOSAICKING CODE CODE DESCRIPTION THE