research in the nhs: hr good practice resource pack frequently asked questions: human resources and contractual arrangements
Research in the NHS:
HR Good Practice Resource Pack
Frequently Asked Questions:
Human Resources and Contractual Arrangements
Human Resources (HR) and contractual arrangements
1.
The HR Good Practice Resource Pack states that honorary research
contracts (HRCs) should only be issued when the researcher’s
activity has a direct bearing on the quality of patient care. What
does this mean?
Researchers require an HRC only if:
*
the research is hosted in the NHS and
*
they have no contractual relationship with the NHS and
*
the activities which the researcher plans to undertake in the host
NHS site involve interacting with individual patients in a way
that has a direct bearing on the quality of their care.
A researcher’s activity may be deemed to have a direct bearing on the
quality of patient care if:
*
the researcher’s activity could directly influence decisions made
on:
*
a patient’s access to care
*
the care pathway which the patient followed
*
the type, quality or extent of prevention, diagnosis or
treatment of illness.
*
the researcher’s action could foreseeably cause injury or loss to
patients or service users to whom the NHS organisation has a duty
of care, leading to a possible case of clinical negligence being
made against the NHS organisation.
Examples of activities that could have a direct impact on care
include:
*
taking consent for an interventional study (as this will determine
an individual's access to a specific treatment)
*
delivering a treatment that forms part of the research study
*
performing phlebotomy on trial patients (as this is an invasive
procedure which could lead to injury or infection).
Activities that would not have a direct impact on care include:
*
some types of interview study, where information from the study
will NOT feed into the patient’s care plan or decision-making in
relation to the care of the patient
*
undertaking the randomisation procedure to allocate trial patients
to a specific treatment, as this is preceded by the consent
process. Randomisation is considered a research procedure.
By issuing an HRC, the NHS organisation ensures that:
1.
Patients in receipt of these research procedures come under the
NHS duty of care and the NHS indemnity scheme applies to the
researchers and their activities.
2.
The researcher is accountable to the NHS organisation for their
activities, explicit supervision arrangements are in place, and
the researcher is made aware of the relevant NHS policies with
which they must comply in their activities.
3.
The researcher’s substantive employer understands the research
activities and has undertaken all the necessary checks in relation
to the researcher’s suitability to carry out those activities.
2.
Shouldn’t we issue an HRC to all researchers to make sure that we
have a way of controlling their activities so that we minimise the
risk to the NHS?
The Department of Health (DH) previously advised that researchers who
do not have a substantive contract with an NHS body, but whose
research involves NHS staff or patients, their organs, tissue or data,
should have an honorary contract with an NHS body. The advice was
withdrawn some years ago.
The UK Research Governance Frameworks make it clear that appropriate
allocation of responsibility, and hence liability, is fundamental to
good overall governance of research. Higher Education Institutions
(HEIs) must accept their responsibilities as employers of researchers,
because it is only the employer that can be responsible ultimately for
ensuring the suitability of the individual in terms of training,
experience and conduct.
HEI substantive employers must retain accountability and liability for
the actions of researchers that are outside the core responsibility of
NHS organisations, i.e. actions that do not relate to the legal duty
of quality and the common law duty of care of the NHS organisation.
NHS organisations should not lay claim to responsibility, and hence
liability, for issues that are outside their ability to fully
discharge.
HEIs, as employers, are responsible for ensuring that staff conducting
research within the NHS have been subject to appropriate
pre-engagement checks commensurate with their proposed research
activity in the NHS (and in line with NHS standards in this area), are
appropriately trained to carry out their research activity and to
handle confidential information, and that disciplinary arrangements
are in place for handling breaches of confidentiality.
The Research Passport system provides for information-sharing between
the NHS organisation and the substantive HEI employer to ensure that
overall governance arrangements are in place to support researchers
and their activity.
3.
In what circumstances should a Letter of Access (LoA) be issued
instead of an HRC?
The HR Good Practice Resource Pack contains three types of LoAs, these
should be issued in the following circumstances:
For HEI researchers, the Example letter of access for university
researchers who do not require an HRC (DOC) should be issued in the
following circumstances:
*
where the research is hosted by the NHS and
*
the researcher needs to access NHS facilities or patients or data
to undertake their research and
*
it is judged that the researcher’s activity will not have a direct
bearing on patient care.
For HEI researchers, the Example letter for university researchers,
where the NHS site accepts an existing HRC should be issued in the
following circumstances:
*
where the lead NHS organisation has already validated a Research
Passport form, and
*
the lead NHS organisation judged that the research activity had a
direct bearing on patient care, and
*
the lead NHS organisation issued an HRC, and
*
the researcher now needs to undertake the same research activity
in a different NHS organisation.
For NHS researchers and clinical academics1 the Example NHS to NHS LoA
(DOC) should be issued following the procedures outlined below
*
Researchers with an existing substantive NHS or honorary clinical
contract do not require an HRC
*
The NHS organisation which hosts their research can accept their
existing relationship with the NHS
*
The NHS to NHS LoA gives permission for any types of research
activity to be undertaken at the host site
*
The researcher’s substantive NHS employer remains fully
responsible for undertaking and updating the pre-engagement
checks.
The procedure for researchers who require an NHS to NHS LoA has been
revised as follows:
A new NHS to NHS (LoA) – proforma confirmation of pre-engagement
checks has been developed. The R&D office for the employing Trust is
responsible for ensuring this confirmation is issued by their Trust
for all staff requiring NHS to NHS LoAs. For clinical academics, the
Trust holding the honorary clinical contract may need to liaise with
the HEI who is the substantive employer, in order to issue the signed
proforma.
The NHS researcher/clinical academic can then submit this declaration,
along with their CV, to all NHS organisations who are hosting their
research. The host site can accept this declaration as confirmation
that the required pre-engagement checks have been undertaken by the
employer and issue the Example NHS to NHS LoA (DOC) to the researcher,
copied to the researcher’s substantive employer. The copy NHS to NHS
LoA sent to the employer will also be accompanied by a copy of the
proforma confirming completion of pre-engagement checks submitted to
the host organisation.
4.
Who needs a Research Passport?
If you have no contractual relationship with the NHS, you may need a
Research Passport, which enables the NHS to decide whether or not you
need an HRC or LoA to enable you to undertake your research within NHS
facilities. A Research Passport is valid for a period of three years
and may be project-specific or may cover a number of projects. The
Research Passport may be used for both single-site and multi-site
studies.
5.
Why is the Research Passport form not completed for NHS employees
who need to carry out their research activity in a different NHS
Trust?
Throughout the NHS there is a level of assurance with regard to the
standards and the processes in place to support HR arrangements as
applied at individual NHS organisations. With the introduction of the
Vetting and Barring Scheme (VBS), the employer as the Regulated
Activity Provider is responsible for the management and control of
that person’s activity, and for making the arrangements for their
employees to work in that activity.
The NHS Employment Check Standards2 have been developed with key
stakeholders including the DH, the Centre for the Protection of the
National Infrastructure (CPNI), and employers in the NHS, and includes
all pre-appointment checks that are required by law, those that are
mandated by DH policy, and those that are required for access to the
NHS Care Record Service. Furthermore, employer organisations in the
NHS need to evidence their compliance of these standards as part of
the Care Quality Commission's (CQC) Annual Health Check (formerly the
Healthcare Commission).
In addition, there are existing processes and systems in place to
facilitate the movement of highly mobile staff within the NHS as a
whole, for example from October 2009, the Electronic Staff Record
(ESR) contains details of pre-engagement occupational health screening
and has replaced the Occupational Health Smart Card (OHSC) system. On
this basis, the advice in the HR Good Practice Resource Pack in
relation to those with a substantive or honorary clinical NHS contract
is that the Research Passport system is not required when sharing
information.
The NHS to NHS LoA should be issued, which:
*
accepts the employee's existing NHS contract
*
accepts confirmation from the substantive employer that the
pre-engagement checks and associated contractual obligations are
in place (through receipt of the NHS to NHS LoA – proforma
confirmation of pre-engagement checks)
*
places the obligation fully on the substantive NHS employer to
ensure these checks continue to be in place for the role to be
undertaken in the NHS organisation hosting the employee’s research
activity.
6.
Does the letter of agreement between NHS organisations HAVE to be
in place between NHS organisations, to support NHS to NHS LoAs?
Where NHS organisations can demonstrate that they are effectively
managing and implementing the Research Passport system within the UK
Clinical Research Networks, then it is not necessary to put an
additional letter of agreement between NHS organisations in place to
cover cross-institutional working between the organisations. In
England, NHS organisations can check through their NIHR Comprehensive
Local Research Networks whether individual NHS organisations are
implementing the Research Passport system. In Scotland, Wales and
Northern Ireland, NHS organisations can contact the nominated Research
Passport lead from their Health Department3. Where there is any doubt
with regard to the substantive employer's compliance with the scheme,
host organisations can issue the letter of joint arrangements
alongside the NHS to NHS LoA.
7.
The Research Passport form does not provide the relevant
information relating to the start and end-date of a researcher’s
substantive contract. How can I ensure that the duration of the
Research Passport and the associated HRC or LoA does not run
beyond a researcher’s contract end-date?
The Research Passport form (Version 2) has been amended to include
this information. For NHS to NHS arrangements the NHS to NHS LoA –
proforma confirmation of pre-engagement checks also requires that the
substantive employer confirms the contract end-date. Working with the
current application form (Research Passport form (Version 1), there
are a number of additional principles and safeguards available to
address this issue:
1) The substantive employer is responsible for the actions of their
employee
In order to fulfil this responsibility, the substantive employer
should ensure that they have established an appropriate process for
notifying and monitoring changes to the content of their employee's
Research Passport (or the conditions of their employment for NHS staff
and clinical academics) and that this process is documented in the
organisation's procedures and policies. The CLRNs in England and the
Health Departments of the Devolved Nations are working with academic
and healthcare organisations to ensure that substantive employers are
aware of this requirement.
2) The Research Passport holder is obliged to notify Trusts of changes
in their situation
Similarly, the substantive employer should ensure that they have
established an appropriate process to actively manage the researchers
they employ. In addition, HRCs and LoAs place an obligation on the
researcher to provide details of any changes to the terms on which the
HRC or LoA was issued to the host organisation.
3) Limit the dates of the Research Passport or HRC to coincide with
the substantive contract
The organisation validating the Research Passport can contact the
researcher or their employer to confirm the contract details and where
the duration of an applicant’s substantive contract is less than the
duration of the project, organisations may choose to limit the dates
of the validity of a Research Passport (and any HRC or LoA issued
following the validation of a Research Passport) or stipulate a review
date to coincide with the terms of the substantive contract. The
latter would enable an extension or new HRC/LoA to be issued if
appropriate, and would ensure appropriate tracking. A similar approach
can be applied to researchers working under NHS to NHS arrangements.
8.
How does the Research Passport system apply to the staff who work
for Independent Contractors, for example GPs or practice nurses,
when they are carrying out research in the NHS?
A PCT, like all NHS Trusts, has a duty of care to its patients,
including those who are cared for by a GP. Therefore a PCT retains
vicarious liability for patients who receive care from a GP or their
staff, and for patients who are participants in research undertaken
during provision of NHS services through their GP. Although the new
NHS Contract4 defines staff employed by Independent Contractors (e.g.
practice nurses) as NHS staff, NHS indemnity arrangements (the
Clinical Negligence Scheme of Trusts (CNST)) specifically do not
extend to Independent Contractors (or their staff) working under
contract for services to a PCT. Therefore, the issuing of an NHS
honorary contract to this group of practitioners does not bring them
within the scope of NHS indemnity arrangements.
This means that PCTs should not extend HRCs to practice staff if they
are conducting research as part of their NHS practice. However, the
PCT may wish to highlight that compliance with research governance is
a contractual obligation for such staff as part of the broader
obligation to comply with the PCT's corporate risk management
arrangements.
Medical defence organisations (MDOs) provide cover for clinical
negligence to GPs for the service they (and their staff) are
contracted to provide to the NHS. If they undertake research as part
of their routine activity, their professional indemnity arrangements
would normally provide adequate cover for that activity. However, this
should be confirmed with their MDO before starting the research5.
If a GP or practice nurse is supporting research activity that is not
considered part of the services under the NHS contract, the PCT will
need to ascertain whether it ows a duty of care to the study
participants with respect to the research activity. If this is the
case, then the GP or practice nurse is operating within the vicarious
liability of the PCT and arrangements should be made to include these
staff under CNST arrangements. Such arrangements would involve the
organisation responsible for the research, either the PCT or HEI,
putting in place a contract for services with the GP or practice nurse
relating to these additional research activities6. If the organisation
responsible is the HEI, then the Research Passport system could then
be used to obtain an HRC or LoA as applicable. If the organisation
responsible is the PCT, the PCT is encouraged to accept the
pre-engagement checks undertaken by the GP practice, though it can
request its own pre-engagement checks if warranted following a
proportionate risk assessment. NHS to NHS arrangements as described in
the HR Good Practice Resource Pack would then cover any additional
movement of these staff to other participating NHS organisations.
Where the research activity and involvement of the GP or practice
nurse is commercially funded, the commercial company should hold
adequate insurance to indemnify the research, covering negligent and
non-negligent harm and provide this cover for the GP/practice nurse.
Although the specific example used in this guidance refers to GPs and
practice nurses, the same also applies to other Independent
Contractors based in primary care including dentists,
ophthalmologists, community pharmacists and podiatrists.
9.
How does the Research Passport system apply to students?
The research activities of undergraduate and postgraduate students who
conduct research as part of their healthcare placements will come
under the memorandum of understanding between the HEI and the NHS
organisation which governs healthcare placements. This confirms the
accountability arrangements between the organisations. Students on
healthcare placements also have the appropriate pre-engagement checks
conducted before they can commence a course which includes a
healthcare placement7, 8. NHS organisations can delegate the
verification of identity and issue of a Smartcard (within the
Registration Authority governance framework) to educational
establishments. Any research conducted as part of healthcare
placements should come under the existing arrangements for such
students. Therefore students conducting research as part of their
healthcare placements should not be issued with HRCs or letters of
access by the NHS organisation.
Where students are undertaking research as part of a Masters or PhD,
and that course is not subject to a healthcare placement agreement
with clinical supervision, then the Research Passport system should be
applied. For students, the HEI would need to ensure that the student
admissions section was able to complete the sections of the Research
Passport normally completed by HR for employed staff. On submission of
a complete and validated Research Passport, the NHS organisation may
issue the student with an HRC or LoA, depending on the nature of the
research activity.
10.
How does the Research Passport apply to visiting international
researchers that are not formally employed by the hosting
university?
In situations such as these, the university hosting the researcher
would need to complete the Research Passport form. Where there is no
formal employment contract in place between the researcher and the
hosting university, a suitable honorary academic contract should be
put in place to ensure that there is clear accountability between the
hosting university and the researcher.
The hosting university should then undertake the necessary
pre-engagement checks to support the completion of the Research
Passport. Any associated cost would be allocated in accordance with
local policy and the researcher’s funding source.
Where an international researcher presents occupational evidence from
their country of origin, the hosting university would need to assess
the evidence, bearing in mind any differences in clinical or
regulatory practice compared with the UK, and determine whether this
evidence could be used to support completion of the Research Passport
form. Checks should be repeated where considered necessary.
The completed Research Passport would be presented to the Lead NHS
organisation for validation in the usual manner.
11.
How does the Research Passport system apply to trial data
monitors?
It is the role of the sponsor (and the data monitor’s employer, if
different) to take responsibility for the actions of any trial data
monitor. The suite of model agreements for use in both commercial and
non-commercial clinical trials provides specific information relating
to the activities of trial data monitors including issues of access
and confidentiality. For trial data monitors, a clinical trial
agreement should be put in place addressing the issues relating to
their activities and a Research Passport and subsequent HRC or LoA
should not be used.
Any NHS organisation that has any concerns about the conduct of a
trial data monitor must inform the sponsor (and their employer, if
different), as this may be a matter of misconduct that the employer
must follow up.
12.
How does the Research Passport system apply to staff such as
research nurses provided to the NHS Trust by Contract Research
Organisations (CROs), to undertake research activities in the
Trust which form part of a commercial study
In commercially sponsored trials, commercial research staff should not
be given a Research Passport, or issued with an HRC or LoA or any
other document that could be construed as indicating that the NHS
organisation is accepting liability for their actions.
With the exception of clinical study data monitors, the UKCRC model
agreements do not cover issues relating to commercial organisations
providing staff to undertake commercial research activities in the
host NHS site (e.g. commercial research nurses). Therefore, the host
organisation needs to ensure that a contract for the provision of
these services is put in place with the commercial organisation. This
contract should address all issues relating to the activities and
suitability of the commercial staff (e.g. pre-engagement check
requirements (Criminal Records Bureau (CRB), occupational health,
professional registration, right-to work, qualification etc),
training, accountability and management arrangements, insurance for
negligent actions etc).
The host organisation's legal, HR or procurement departments may be
familiar with standard contracts for services that address these
issues and may have appropriate templates available for use in these
circumstances. In many cases, the CRO may provide a template agreement
that can be amended by your Trust as appropriate.
Please also see guidance under QUESTION 39 of the Frequently Asked
Questions Full Supplement.
13.
How does the Research Passport system apply to contract research
staff who undertake research activities forming part of a
non-commercial study sponsored either by the university, the NHS
or a non-commercial funder?
There is a distinction to be made when the research is being sponsored
and led by a non-commercial organisation, compared with a situation
when the study is wholly commercially sponsored. In the former
situation the CRO staff are being contracted by the university to
undertake non-commercial research. Therefore the university should
ensure it has appropriate agreements in place with the CRO governing
the provision of services for the study, which appropriately address
indemnity issues and the requirement for and sharing of information on
pre-engagement checks, where both organisations agree to follow the
requirements of the HR Good Practice Resource Pack. On this basis, the
university can then provide assurance to the Trust about the
suitability of these individuals, and the Trust can deal with these
individuals as though they were university researchers.
The Implementation Project and the HR Good Practice Resource Pack
present a risk management framework to support the decision to accept
pre-engagement checks undertaken by others. In practice, the
university could complete a Research Passport form for the nurse. If
they felt it was appropriate, they could use evidence of
pre-engagement checks undertaken by the CRO to support this
application. (NB where the research involves regulated activity, the
HEI would need to initiate Independent Safeguarding Authority (ISA)
registration and register their interest in the individual concerned –
see section on VBS below). Similarly, the Trust receiving the Research
Passport should apply an appropriate and proportionate risk assessment
to the evidence provided in deciding whether it is comfortable
accepting these checks. If risk assessment demonstrates that it is
warranted, the Trust can request that the University repeats the
checks undertaken by the CRO or the Trust can request its own.
Depending on the nature of the clinical trial and the activity
involved, an HRC or LoA may be issued. The RP issued by the university
and the HRC or LoA issued by the NHS would be project-specific and
time-limited.
14.
Bank nurses seem to present some considerable challenges to the
Research Passport system. Is there any national guidance on bank
nurses?
With regard to the handling of bank nurses, if the nurses are
recruited to the bank in accordance with current NHS Employment Checks
Standards9, and are then involved in research roles as NHS staff,
these bank staff can be handled using the NHS to NHS arrangements
described in the HR Good Practice Resource Pack. They should not use
the Research Passport form.
If, however, bank nurses have an HEI as their substantive employer,
the Research Passport system will apply. The Research Passport form
includes an Appendix which allows new projects to be added, or
additional information regarding amendments to agreed research
activities to be documented.
15.
Can a project-specific passport be converted into to a
multi-project/generic passport?
The ideal situation would be for researchers to apply for the
multi-project/generic three-year passport, rather than the
project-specific Research Passport at the outset. However, researchers
in possession of a valid project-specific Research Passport, who then
find that they need to work on more than one project, can at a later
stage submit an amendment to their project-specific Research Passport,
by completing the relevant details in the Appendix to the application
form and submitting this to the Lead NHS organisation for approval.
The Lead NHS organisation then needs to ensure that the pre-engagement
checks in place are also valid for the new activity. If so, the Lead
NHS organisation can convert the project-specific Research Passport
into a multi-project/generic three-year Research Passport. In
addition, the researcher is required to inform all NHS organisations
that have received their original Research Passport of any changes to
information within the Research Passport. Having alerted the other NHS
organisations of the change in their Research Passport from
project-specific to multi/project generic, the researcher can then
provide details of additional studies and/sites as would be the case
for a multi-project/generic three year Research Passport
16.
A researcher with an NHS substantive contract wishes to carry out
research in a different Trust, but their NHS employer states that
they cannot be responsible for this person’s research activity.
What can be done in this circumstance?
In a case such as this, it is important to determine if the researcher
is undertaking this activity as part of their contracted working
hours/job description, or whether it is completely outside their
contracted responsibilities. This issue needs to be clarified and
agreed between the employer, employee and the NHS Trust hosting the
research, in order to enable the appropriate arrangements to be put in
place.
If the activity is being undertaken as part of the researcher's
substantive contracted hours and job role, and they are being paid by
their substantive employer whilst completing this activity, the
substantive employer will remain responsible for the researcher's
activity, as they will continue to have a duty of care towards their
employee even if the research is not directly related to the
substantive Trust. In this case, the NHS to NHS agreements described
in Question 3 (above) and the HR Good Practice Resource Pack should be
followed, and the NHS to NHS LoA should be issued by the host NHS
Trust to the researcher and their NHS employer.
If the researcher is undertaking this activity independently and not
in the course of their paid employment, they should ensure that they
have appropriate professional indemnity in place to cover the research
activity. Evidence of this would need to be reviewed as part of the
research governance review and a suitable agreement put in place
between the Trust hosting the research and the researcher (e.g. a
contract for services).
If the researcher is undertaking this activity completely outside
their contracted hours, the Trust hosting the research may enter a
separate contract with this researcher enabling them to undertake
research sessions in the host Trust. In this case, the host Trust
becomes responsible for the researcher's activity and would be
encouraged to accept the existing pre-engagement checks undertaken by
the substantive employer following a proportionate risk assessment
rather than undertaking its own.
It should be noted that if the host Trust issues an HRC, then
liability for the researcher’s activity is transferred from the
employing Trust to the host Trust.
17.
Does the Research Passport system apply to researchers who are not
employed either by the NHS or by a university (e.g staff employed
by social care organisations or the voluntary sector)?
In this situation it is important firstly to clarify that the research
is hosted through the NHS. IF so, it is then important to establish to
whom the researcher is affiliated in respect of the research activity
being undertaken. For example, in an HEI sponsored non-commercial
trial, if the HEI sponsor sub-contracts staff to assist in the
delivery of the research, the organisation responsible for the
research, i.e. the HEI, should put in place a contract with the
individual(s) relating to these additional research activities. Once
this is done, the Research Passport system can be used to obtain an
HRC or LoA as applicable. Similarly, if an NHS Trust is responsible
for the conduct of the study, they could put in place a contract for
the additional research activities with the researcher and then NHS to
NHS arrangements would apply.
Where individuals are employed by organisations which have no formal
agreements in place to operate the Research Passport system, and
therefore there is no established method for sharing information, NHS
organisations hosting research may need to undertake appropriate
checks on the researchers and claim the costs of such checks from the
employer or research funder.
NHS organisations can establish local arrangements with other
organisations in addition to HEIs, e.g. public sector research
partners such as local authorities or charities, where those
organisations can also undertake to implement the Research Passport
system, in order to facilitate methods for sharing information on
researchers where their research is hosted through the NHS.
Please also see guidance under QUESTION 10 for commercially sponsored
research and QUESTION 13 for Bank Nurses.
List of Abbreviations
CLRN Comprehensive Local Research Network
CPNI Centre for the Protection of National Infrastructure
CQC Care Quality Commission
CNST Clinical Negligence Scheme for Trusts
CRO Contract Research Organisation
CRB Criminal Records Bureau
DH Department of Health, England
ESR Electronic Staff Record
GP General Practitioner
HEI Higher Education Institution
HRC Honorary Research Contract
HR Human Resources
ISA Independent Safeguarding Authority
LoA Letter of Access
MDO Medical Defence Organisation
NHS National Health Service
OHSC Occupational Health Smart Card
PhD Doctor of Philosophy
PCT Primary Care Trust
UKCRC UK Clinical Research Collaboration
VBS Vetting and Barring Scheme
References
1.
A Review of Appraisal, Disciplinary and Reporting Arrangements for
Senior NHS and University Staff with Academic and Clinical Duties
Department for Education and Skills, September 2001
http://www.academicmedicine.ac.uk/uploads/folletreview.pdf
2.
Annex F3, Delivering Investment in General Practice. Implementing
the new GMS contract Department of Health, December 2003
http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4070242
3.
Indemnity arrangements within Primary Care – who is responsible
for what?
NHS R&D Forum, Primary Care Working Party
http://www.rdforum.nhs.uk/workgroups/primary/indemnity_arrangements.doc
4.
Medical student access to patient records
NHS Employers
http://www.nhsemployers.org/PlanningYourWorkforce/MedicalWorkforce/Medical_Education_and_training/Pages/MedicalstudentsCRS.aspx
5.
NHS Employers' Check Standards
http://www.nhsemployers.org/RECRUITMENTANDRETENTION/EMPLOYMENT-CHECKS/EMPLOYMENT-CHECK-STANDARDS/Pages/Employment-Check-Standards.aspx
6.
Research in the NHS: indemnity arrangements
Gateway reference: 5957
Department of Health, December 2005
http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4125281
1 Clinical academics hold joint appointments across NHS and HEI
organisations to conduct clinical activity, teaching and research.
http://www.academicmedicine.ac.uk/uploads/folletreview.pdf
2 NHS Employers Check Standards
http://www.nhsemployers.org/RECRUITMENTANDRETENTION/EMPLOYMENT-CHECKS/EMPLOYMENT-CHECK-STANDARDS/Pages/Employment-Check-Standards.aspx
3 http://www.ukcrc.org/regulationgovernance/researchpassport/
4 Annex F3, Delivering Investment in General Practice. Implementing
the new GMS contract Department of Health, December 2003
http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4070242
5 Research in the NHS: indemnity arrangements
Gateway reference: 5957
Department of Health, December 2005
http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4125281
6 Indemnity arrangements within Primary Care – who is responsible for
what?
NHS R&D Forum, Primary Care Working Party
http://www.rdforum.nhs.uk/workgroups/primary/indemnity_arrangements.doc
7 NHS Employers' Check Standards
http://www.nhsemployers.org/RecruitmentAndRetention/Employment-checks/Employment-Check-Standards/Pages/Employment-Check-Standards.aspx
8 Medical student access to patient records
NHS Employers
http://www.nhsemployers.org/PlanningYourWorkforce/MedicalWorkforce/Medical_Education_and_training/Pages/MedicalstudentsCRS.aspx
9 NHS Employers' Check Standards
http://www.nhsemployers.org/RECRUITMENTANDRETENTION/EMPLOYMENT-CHECKS/EMPLOYMENT-CHECK-STANDARDS/Pages/Employment-Check-Standards.aspx
Version 1.0, February 2010
 SOLICITUD Y ENTREGA DE DOCUMENTO LIBERACIÓN DE GARANTÍA
SOLICITUD Y ENTREGA DE DOCUMENTO LIBERACIÓN DE GARANTÍA UNIVERSITAS GUNADARMA SK NO 92 DIKTI KEP
UNIVERSITAS GUNADARMA SK NO 92 DIKTI KEP IMPRESO DE SOLICITUD APPLICATION FORM CURSO ACADÉMICO
IMPRESO DE SOLICITUD APPLICATION FORM CURSO ACADÉMICO  USING CATKIN V203 THE RELATIVISTIC KINEMATICS PROGRAM IN EXCEL
USING CATKIN V203 THE RELATIVISTIC KINEMATICS PROGRAM IN EXCEL ACPWG N WP6FL01 ISSUE 02 DRAFT FLIMSY ON FUTURE
ACPWG N WP6FL01 ISSUE 02 DRAFT FLIMSY ON FUTURE AUTUMN 1 YEAR 9 WEEK 4 LESSON 2 END
AUTUMN 1 YEAR 9 WEEK 4 LESSON 2 END PERFILADO Y TEXTURIZADO DEL PAVIMENTO HIDRÁULICO DEL KM 40+000
PERFILADO Y TEXTURIZADO DEL PAVIMENTO HIDRÁULICO DEL KM 40+000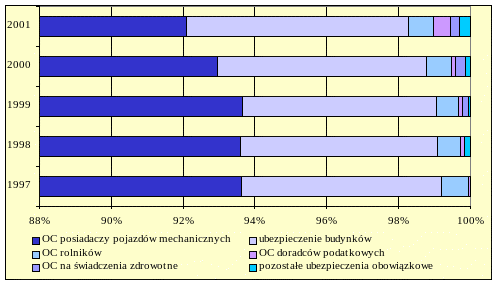 VIIIIANALIZA WYBRANYCH SEGMENTÓW RYNKU UBEZPIECZEŃ IX1 ANALIZA UBEZPIECZEŃ
VIIIIANALIZA WYBRANYCH SEGMENTÓW RYNKU UBEZPIECZEŃ IX1 ANALIZA UBEZPIECZEŃ NAME DATE DIRECTIONS COMPLETE THE VENN DIAGRAM BELOW
NAME DATE DIRECTIONS COMPLETE THE VENN DIAGRAM BELOW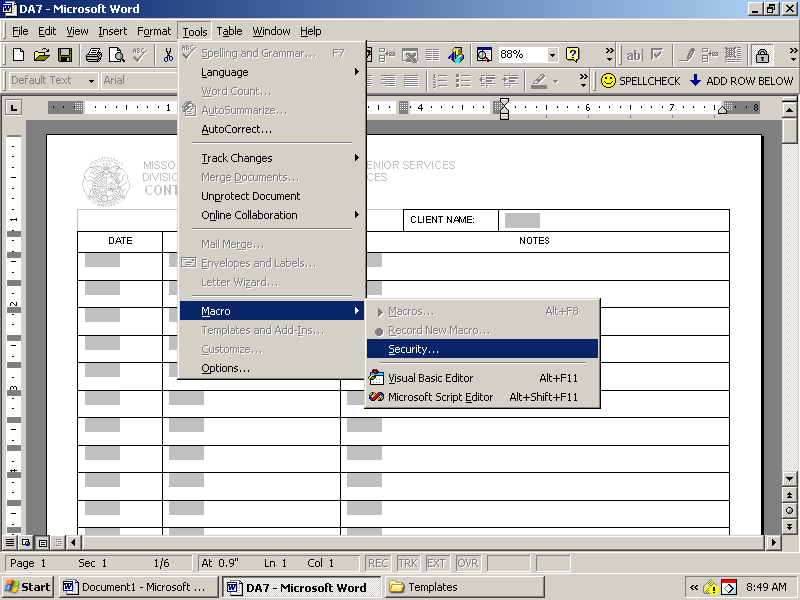 DOWNLOAD THE DA7 FROM EITHER THE CASE MANAGEMENT OR
DOWNLOAD THE DA7 FROM EITHER THE CASE MANAGEMENT OR DEPARTMENT OF CIVIL ENVIRONMENTAL & GEOMATICS ENGINEERING DE MEEROFF
DEPARTMENT OF CIVIL ENVIRONMENTAL & GEOMATICS ENGINEERING DE MEEROFF LEY FEDERAL DE SEGURIDAD PRIVADA CÁMARA DE DIPUTADOS DEL
LEY FEDERAL DE SEGURIDAD PRIVADA CÁMARA DE DIPUTADOS DEL