ideal gas law ex-9954 page 7 of 7 ideal gas law equipment required: ideal gas law syringe td-8596 pressure/temperatur
Ideal Gas Law EX-9954 Page 7 of 7
Ideal Gas Law
EQUIPMENT
REQUIRED:
Ideal Gas Law Syringe
TD-8596
Pressure/Temperature Sensor
PS-2146
Absolute Zero Apparatus
TD-8595
Plastic Containers
740-183
NOT INCLUDED, BUT REQUIRED:
Hot Water
Cold Water or Ice
Calipers
INTRODUCTION
The temperature, volume, and pressure of a gas are measured
simultaneously to show that they change according to the Ideal Gas
Law. Special cases of constant volume, constant temperature, and
adiabatic are also investigated.
THEORY
In 1662, Robert Boyle discovered that the product of the pressure (P)
and volume (V) of a gas at a constant temperature is constant.
PV = k1
Where, k1 is a constant.
Therefore, the pressure and volume are inversely proportional.
In the 1787, Jacques Charles experimentally verified that the volume
and temperature (T) of a gas at constant pressure are directly
proportional.
V=Tk2
Where, k2 is a constant.
In 1802, Joseph Gay-Lussac discovered the direct relationship between
the pressure and temperature of a gas at constant volume:
P=Tk3
Where, k3 is a constant.
The Ideal Gas Law combines the three discoveries above. It relates the
absolute pressure (P) and volume (V) of a gas to the absolute
temperature (T) in degrees Kelvin.
PV=nRT
where n is the number of moles of gas, and R is the ideal gas
constant.
Part I: Ideal Gas Law Syringe
SETUP
T he Ideal Gas Law Syringe allows simultaneous measurements of
temperature and pressure of a gas as it is compressed. The mini stereo
jack is connected to a low thermal mass thermistor built into the end
of the syringe to measure temperature changes inside the syringe. The
mini stereo jack plugs directly into the sensor.
T he white plastic tubing coupler attaches to the pressure port
of the sensor: A slight twisting motion locks the coupler onto the
port. This white plastic connector can be disconnected and
re-connected during the experiment to allow for different initial
plunger positions. All of the clear plastic fittings are glued in
place and cannot be removed.
The plunger is equipped with a mechanical stop that protects the
thermistor, and also allows for a quick, predetermined change in
volume. Never slam the plunger down on the table. Always grip the
syringe and plunger as shown to compress the air.
PROCEDURE
1. In DataStudio, construct a graph of Absolute Pressure (kPa) vs Time
and Temperature (K) vs. Time. Set the sample rate to 20 Hz.
2. Disconnect the white plastic pressure coupler from the sensor.
Press the plunger of the syringe all the way in until the handle of
the plunger bottoms out on the mechanical stop. Record this minimum
volume: It should be close to 20 cc.
3. Set the plunger at 40 cc, and then re-connect the coupler to the
sensor.
4. Start recording data. Quickly compress the plunger all the way in,
and keep it compressed. The plunger handle should be bottomed out
against the mechanical stop.
5. Watch the graphs of pressure and temperature, and continue to hold
the plunger in until the values are no longer changing. This should
take around 10 seconds.
6. After the temperature and pressure have equalized, release the
plunger. Again, watch the graphs and wait until the values are no
longer changing.
7. Stop data collection.
ANALYSIS
1. Look at your pressure and temperature graphs. Correlate the changes
in pressure and temperature to the movement of the plunger.
2. What happened to the temperature when the air was compressed? Why?
3. What is the equilibrium temperature of the gas when it was
compressed? Why? What is the equilibrium pressure? Why does it not go
back to “room pressure”?
4. What happened to the temperature during the expansion (when you
released the plunger)? Why? Does it go below room temperature? Does
the pressure go below “room pressure”? What would you have to do to
make this happen?
5. Create a Data Table in DataStudio of the Pressure and Temperature
data.
6. Measure the initial temperature (T1) and pressure (P1) of the gas
from your data just before you compressed it. You can highlight an
area (click and drag) in the graph and that data will appear in the
data table. This data corresponds to an initial volume (V1) of 40cc.
7. Highlight the area on the temperature graph where it peaks. Pick
the place where the temperature has peaked, not the pressure. It takes
the temperature sensor about 1/2 second to respond. Record the peak
temperature (T2) and the corresponding pressure (P2) for that time.
You want two values that occurred at the same time. This data
corresponds to the volume (V2) of 20cc. Note: If the compressed volume
marked on the syringe is different than 20 cc, use that value instead.
8. Use the Ideal Gas Law to show that the ratio of volumes can be
expressed as
where the subscript 1 refers to the initial state (volume = 40 cc) and
the subscript 2 refers to the final state (volume = 20 cc) after
compression.
9. Use your values of pressure and temperature to calculate the ratio
of volumes. How does this compare to the actual ratio? Are they about
the same?
Part II: Constant Temperature
PROCEDURE
1. Add a digits display of temperature in DataStudio.
2. Disconnect the white plastic pressure coupler from the sensor. Set
the plunger at 45 cc, and then re-connect the coupler to the sensor.
3. Start recording data. Compress the plunger to 40 cc and hold it at
this position. Watch the temperature on the digits display and wait
until it has dropped down to close to room temperature. Note the final
temperature. Each time you compress the air in this sequence, wait
until the temperature returns back down close to this value.
4. Compress the plunger to 35 cc and hold it at this position. Watch
the temperature, and hold the plunger at 35 cc until the temperature
has dropped to the value you noted in step 3. Do not release the
plunger.
5. Compress the plunger to 30 cc, and wait until the temperature drops
as before.
6. Repeat for 25 cc and 20 cc.
7. Stop recording data.
ANALYSIS
1. Look at your pressure and temperature graphs. Correlate the changes
in pressure and temperature to the movement of the plunger.
2. Highlight the area on the graph when the plunger was at 40 cc. Use
the data table to determine the equilibrium pressure and temperature.
3. Repeat for all other volumes. For each position, pick the pressure
that has the temperature closest to the “equilibrium” value. It does
not matter what this temperature is, as long as all pressures are
measured at the same temperature. Record all your values in a table.
4. For each of the pressures, calculate the inverse pressure (1/P).
Graph Volume vs 1/P. Why does this give a straight line? Use the Ideal
gas law to show that a graph of Volume vs 1/P results in a straight
line with a slope given by
Slope = nRT
5. Determine the slope of this line from your graph of Volume vs. 1/P.
Use your values to determine the number of moles (n) of air in the
syringe. Pay attention to the units!
6. Look carefully at the graph. Why is there an offset in the axis for
the volume? How do you account for this extra volume?
FURTHER INVESTIGATIONS
1. Disconnect the white plastic pressure coupler from the sensor. Set
the plunger at 60 cc, and then re-connect the coupler to the sensor.
2. Repeat the procedure, taking pressure and temperature data at each
of the volumes (40cc, 35cc, etc.) as you did before.
3. Put this new data on the same graph. Why is this slope different?
Is the volume offset about the same as before?
Part III: Adiabatic Compression
PROCEDURE
1 Set the sample rate at 50 Hz
2. Disconnect the white plastic pressure coupler from the sensor. Set
the plunger at 60 cc, and then re-connect the coupler to the sensor.
3. Start recording data. As quickly as possible, compress the plunger
from 60 cc down to 20 cc. Do this in one quick motion, bottoming out
the piston on the mechanical stop.
4. Stop recording data.
ANALYSIS
1. Measure the initial temperature (T1) and pressure (P1) of the gas
from your data just before you compressed it. This data corresponds to
an initial volume (V1) of 60cc.
2. For an adiabatic compression, the initial pressure and volume (P1,
V1) are related to the final pressure and volume (P2, V2) by
where (gamma) is the ratio of specific heats, and for air has
a value of 1.40. Use your values to calculate the theoretical peak
pressure if the compression was adiabatic.
3. Measure the peak pressure (P2) after compression. Was this truly
adiabatic?
4. Using the Ideal Gas Law, calculate the theoretical peak
temperature.
5. Measure the peak temperature after compression. Why did it not
occur at the same time as the peak pressure? Why is this temperature
so much lower than the theoretical?
Part IV: Constant Volume
SETUP
The Absolute Zero Apparatus consists of a hollow sphere that acts as a
container of constant volume as the apparatus is placed in different
temperature water baths. Plug the mini stereo jack into the
temperature port to measure the temperature using the thermistor
imbedded in the wall of the sphere. Connect the white plastic pressure
coupling to the pressure port to measure the pressure inside the
sphere.
You will need a source of hot water and a source of cold water (or
ice).
PROCEDURE
1. Set the sample rate at 10 Hz. Set up a graph in DataStudio of
pressure vs. temperature. Select Manual Sampling from the sampling
options in the Setup menu. It is also helpful to set up digit displays
of temperature and pressure.
2. Fill one of the plastic containers with enough hot water to cover
the sphere. Use the other container for a supply of cold water (or
ice). Make sure the white plastic pressure coupler is connected to the
sensor before putting the sphere in the hot water.
3. Start data collection. Completely submerge the sphere and wait for
the pressure and temperature to equalize. Click on keep in the top
menu bar to save that data pair.
4. Add cold water (or ice) to decrease the temperature of the water 5
to 10 oC. Stir the water to get an even temperature and click on keep
when the pressure and temperature equalize.
5. Keep decreasing the temperature and taking data until you have the
system as cold as you can get it. You may need to dump out some of
your water.
ANALYSIS
1. Use calipers to measure the diameter of the sphere, and calculate
its volume. Is this measurement more or less than the actual volume of
the sphere? Why?
2. Use the Ideal Gas Law to show that a graph of Pressure vs.
Temperature results in a straight line with a slope given by
3. Determine the slope of this line from the Pressure vs. Temperature
graph. Use your values to determine the number of moles (n) of air in
the sphere. Pay attention to the units!
FURTHER INVESTIGATIONS
1. Re-fill the container with hot water.
2. Disconnect the white plastic pressure coupler from the sensor and
place the sphere in the hot water. Does air flow in or out of the
sphere? Re-connect the coupler to the sensor.
3. Repeat the procedure, taking pressure data at different
temperatures as you did before.
4. Put this new data on the same graph. Why is this slope different?
Calculate the new number of moles of air.
CONCLUSION
1. Describe what happens to the pressure of a gas at constant
temperature when its volume is changed.
2. Describe what happens to the volume of a gas at constant pressure
when its temperature is changed.
3. Describe what happens to the temperature of a gas at constant
volume when its pressure is changed.
Written by Jon Hanks
 HORARIO DE CLASES PRIMER AÑO SEGUNDO SEMESTRE
HORARIO DE CLASES PRIMER AÑO SEGUNDO SEMESTRE 2009 SUNSEEKER PREDATOR 74 “GRACE ISABELLA” BOAT DESCRIPTION THE
2009 SUNSEEKER PREDATOR 74 “GRACE ISABELLA” BOAT DESCRIPTION THE SEXUALIDAD Y AUTISMO INFORME DANES AUTOR DEMETRIOUS HARACOPOS &
SEXUALIDAD Y AUTISMO INFORME DANES AUTOR DEMETRIOUS HARACOPOS & CENA DE GALA PRESIDIDA POR EL EXCMO CONTRALMIRANTE D
CENA DE GALA PRESIDIDA POR EL EXCMO CONTRALMIRANTE D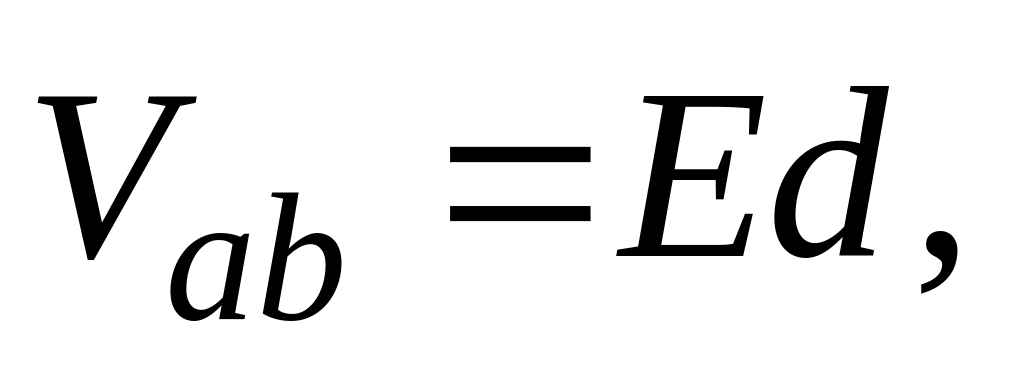 PHYSICS 104 ASSIGNMENT 24 241 IDENTIFY THE CAPACITANCE DEPENDS
PHYSICS 104 ASSIGNMENT 24 241 IDENTIFY THE CAPACITANCE DEPENDS DEPARTEMENT FÜR WIRTSCHAFT SOZIALES UND UMWELT DES KANTONS BASELSTADT
DEPARTEMENT FÜR WIRTSCHAFT SOZIALES UND UMWELT DES KANTONS BASELSTADT SISTEMA NERVIOSO AUTÓNOMO O VEGETATIVO ES EL CONJUNTO
SISTEMA NERVIOSO AUTÓNOMO O VEGETATIVO ES EL CONJUNTO DIRECTIONS TO TUMBLEWEED PARK FROM SCOTTSDALE NORTHEAST VALLEY
DIRECTIONS TO TUMBLEWEED PARK FROM SCOTTSDALE NORTHEAST VALLEY POWERPLUSWATERMARKOBJECT3 L ONDON BOROUGH OF LEWISHAM CORPORATE OH&SMS PROCEDURE
POWERPLUSWATERMARKOBJECT3 L ONDON BOROUGH OF LEWISHAM CORPORATE OH&SMS PROCEDURE EXCMO AYUNTAMIENTO DE SALAMANCA JUNTA ARBITRAL DE CONSUMO ÁRBITROS
EXCMO AYUNTAMIENTO DE SALAMANCA JUNTA ARBITRAL DE CONSUMO ÁRBITROS Spoji Sliku s Početnim Slovom a p s l
Spoji Sliku s Početnim Slovom a p s l