procedure: glucose osr6121, osr6221, and osr6621 this procedure is valid for the following chemistry analyzers: *
Procedure:
GLUCOSE
OSR6121, OSR6221, and OSR6621
This procedure is valid for the following chemistry analyzers:
*
AU400/AU400e
*
AU640/AU640e
*
AU480
*
AU680
*
AU600
*
AU2700/AU5400
Prepared By
Date Adopted
Supersedes Procedure #
Review Date
Revision Date
Signature
Distributed to
# of
Copies
Distributed to
# of
Copies
PRINCIPLE:
==========
Serum glucose levels may be abnormally high (hyperglycemia) or
abnormally low (hypoglycemia)1. Glucose measurements are used in the
diagnosis and treatment of carbohydrate metabolism disorders including
diabetes mellitus, neonatal hypoglycemia, and idiopathic hypoglycemia,
and of pancreatic islet cell carcinoma.
Glucosuria (the presence of urinary glucose) is common in healthy,
pregnant women. The cardinal feature of the glucosuria of pregnancy is
a conspicuous variability both from day to day and during the course
of the day.2 Glucose is not present in normal patient urine.
Determinations of cerebrospinal fluid (CSF) glucose help distinguish
bacterial from viral meningitis; the glucose value is often low (less
than 40% to 45% of simultaneously analyzed, equilibrated serum
glucose) in bacterial meningitis and tuberculous meningitis and is
generally normal in viral disease. Carcinomatous meningitis
(widespread infiltration of the meninges by tumor cells) also drives
CSF glucose values below the normal range.2
INTENDED USE:
=============
System reagent for the quantitative determination of Glucose in human
serum, plasma, urine, or cerebrospinal on Beckman Coulter AU Clinical
Chemistry analyzers.
METHODOLOGY:
============
Stein1 first introduced the hexokinase G-6-PDH method for assay of
glucose in serum or plasma. Several investigators3,4,5,6 have
demonstrated the accuracy and usefulness of the method.
In the Beckman Coulter AU Syste Glucose procedure, glucose is
phosphorylated by hexokinase (HK) in the presence of adenosine
triphosphate (ATP) and magnesium ions to produce glucose-6-phosphate
(G-6-P) and adenosine diphosphate (ADP). Glucose-6-phosphate
dehydrogenase (G6P-DH) specifically oxidizes G-6-P to
6-phosphogluconate with the concurrent reduction of nicotinamide
adenine dinucleotide (NAD+) to nicotinamide adenine dinucleotide,
reduced (NADH). The change in absorbance at 340/380 nm is proportional
to the amount of glucose present in the sample.
HK, Mg2+
Glucose + ATP G-6-P + ADP
G6P-DH
G-6-P + NAD+ 6-Phosphogluconate + NADH + H+
SPECIMEN:
=========
Patient Preparation:
--------------------
Prior to sample collection, an 8-12 hour fast is recommended but not
required for the patient.
Additional instructions for patient preparation as designated by this
laboratory:
Type:
-----
Fasting serum or plasma (EDTA, heparin, or sodium fluoride) samples,
free from hemolysis, are the recommended specimens. Separate from red
cells rapidly to minimize loss of glucose through glycolysis.
Fresh, random collections are recommended for urine specimens.
Additional type conditions as designated by this laboratory:
Handling Conditions:
--------------------
Glucose in serum, free from hemolysis and bacterial contamination, and
without added preservatives, is stable for 8 hours when stored at room
temperature (15-25°C), or 72 hours when stored refrigerated at 2-8°C.7
Fluoride preserved plasma samples are stable for 24 hours at 15-25°C.
Urine specimens should be maintained at 2 - 8°C and analyzed as soon
as possible.7
Cerebrospinal fluid can be stored between 2 - 8°C for at least 5 days
if protected from evaporation. Specimens that will not be tested
within 5 days should be stored frozen at <-20°C immediately after
collection.2
Additional handling conditions as designated by this laboratory:
EQUIPMENT AND MATERIALS:
========================
Equipment:
----------
Beckman Coulter AU400/AU400e, AU480, AU600, AU640/AU640e, AU680,
AU2700, and AU5400 analyzers.
Materials:
----------
Beckman Coulter AU System Glucose Reagent
Final concentration of reactive ingredients:
PIPES- buffer (pH 7.6)
24.0 mmol/L
NAD+
1.32 mmol/L
Hexokinase
0.59 KU/L
ATP
2.0 mmol/L
Mg2+
2.37 mmol/L
G6P- DH
1.58 KU/L
Also contains preservatives.
Reagent storage location in this laboratory:
Test tubes 12 -16 mm in diameter or sample cups (Cat No. AU1063).
Storage location of test tubes or sample cups in this laboratory:
Beckman Coulter Chemistry Calibrator (Cat. No. DR0070)
Beckman Coulter Urine Chemistry Calibrator (Cat. No. DR0090)
Storage location of the calibrator in this laboratory:
Precautions:
1.
For in vitro diagnostic use.
2.
Do not ingest. Harmful if swallowed.
3.
Contains sodium azide as a preservative that may react with lead
joints in copper plumbing to form explosive compounds. Even though
the reagent contains minute quantities of sodium azide, drains
should be well flushed with water when discarding the reagent.
4.
WARNING: POTENTIAL BIOHAZARDOUS MATERIAL. The Chemistry calibrator
(DR0070) and Urine Chemistry calibrator (DR0090) are manufactured
from materials of human origin. No test method can offer complete
assurance that HIV- 1/2, HCV, Hepatitis B, or other infectious
agents are absent from biological materials, all calibrator
material should be handled at the Biosafety Level 2 as recommended
for any infectious human serum or blood specimen in the
CDC/National Institutes of Health manual, Biosafety in
Microbiological and Biomedical Laboratories, 1993.
Preparation:
------------
The Beckman Coulter AU System Glucose Reagent is liquid, ready for
use. No preparation is needed.
The Beckman Coulter Chemistry Calibrator reconstitution:
*
Remove the vials of calibrator and diluent from storage and let
stand at room temperature (18-28C) for 5 minutes.
*
Remove the cap and stopper from the vials of the lyophilized serum
and reconstituting diluent.
*
Using a volumetric pipette or a calibrated air-displacement
pipettor, add exactly 5.0 mL of reconstituting diluent to DR0070
lyophilized serum vial. DO NOT pour directly from the
reconstituting diluent vial.
*
Replace the cap and stopper to the vial of lyophilized serum
immediately after adding the diluent
*
Allow the calibrator to stand for 5-10 minutes. Gently swirl the
contents until completely dissolved.
The Beckman Coulter Urine Chemistry Calibrator is liquid, ready to
use. No preparation is needed.
Storage Requirements:
1. The unopened reagents are stable until the expiration date printed
on the label when stored at 2 - 8°C.
2. Opened reagents are stable for 30 days when stored in the
refrigerated compartment of the analyzer.
3.
Un-reconstituted calibrator and diluent are stable until the
expiration date stated on the label when stored at 2 - 8°C.
4.
For Glucose, reconstituted calibrator materials are stable for 7
days from the date of reconstitution when stored at 2 - 8°C. The
materials should be capped and stored upright 2 - 8°C when not in
use.
5.
Contamination after opening must be avoided.
6.
Urine Chemistry calibrator is stable until the expiration date
printed on the label when stored at 2 - 8°C.
7.
Once opened vials of Urine Chemistry Calibrator are stable for 30
days.
Indications of Deterioration:
Discoloration of the reagent, visible signs of microbial growth,
turbidity or precipitation in reagent may indicate degradation and
warrant discontinuance of use.
Additional storage requirements as designated by this laboratory:
PERFORMANCE PARAMETERS:
=======================
The following data was obtained using this Glucose Reagent on Beckman
Coulter AU analyzers according to established procedures. Results
obtained at individual facilities may differ.
Precision:11
------------
Estimates of precision, based on CLSI recommendations10, are
consistent with typical performance. The within run precision for
serum samples is less than 3%CV and total precision is less than 3%CV.
Assays of control sera were performed and this data reduced following
CLSI guidelines.
SERUM
N=100
Within run
Total
Mean, mg/dL
SD
CV%
SD
CV%
59.1
0.4
0.7
0.9
1.6
258.1
1.0
0.4
3.8
1.5
URINE
N=100
Within run
Total
Mean, mg/dL
SD
CV%
SD
CV%
31.0
0.2
0.6
0.3
1.0
312.8
1.0
0.3
3.3
1.1
CSF(AU400/400e)
N=60
Within run
Total
Mean, mg/dL
SD
CV%
SD
CV%
32.23
0.177
0.55
0.338
1.05
60.20
0.392
0.65
0.712
1.18
CSF (AU600)
N=60
Within run
Total
Mean, mg/dL
SD
CV%
SD
CV%
41.0
0.19
0.5
1.20
2.9
63.0
0.28
0.4
1.68
2.7
-----------
Method Comparison:11
--------------------
Patient samples were used to compare this Glucose Reagent.
Representative performance data on AU analyzers is shown in the next
table.
SERUM
Y Method
AU640/ AU640e
X Method
AU600
Slope
0.986
Intercept
0.4
Correlation Coeff. (r)
1.000
No. of Samples (n)
180
Range (mg/dL)
10 - 644
URINE
Y Method
AU640/ AU640e
X Method
AU600
Slope
0.996
Intercept
0.8
Correlation Coeff. (r)
0.9997
No. of Samples (n)
96
Range (mg/dL)
2 - 1099
Sensitivity:
Typical change in absorbance per minute for 1 mg/dL of Glucose is 2.0
mAbsorbance in the Olympus AU400/AU400e, AU480, AU600, AU640/AU640e,
and AU680 analyzers and 2.5 mAbsorbance in the AU2700, and AU5400
analyzers.
CALIBRATION:
============
Standard Preparation:
---------------------
For Serum: Perform a one-point calibration (AB) using a water blank
(blue rack) and the appropriate calibrator in a yellow calibration
rack. The frequency of calibration is every 30 days. Calibration of
this glucose procedure is accomplished by use of the Beckman Coulter
Chemistry Calibrator (Cat No. DR0070), which is traceable to the
National Institutes of Standards and Technology (NIST) Standard
Reference Material (SRM) 965a.
For Urine: Perform a one-point calibration (AB) using a water blank
(blue rack) and the appropriate calibrator in a yellow calibration
rack. The frequency of calibration is every 30 days. Calibration of
this glucose procedure is accomplished by use of the Beckman Coulter
Urine Chemistry Calibrator (Cat No. DR0090)
For CSF: Perform a one-point calibration (AB) using a water blank
(blue rack) and the appropriate calibrator in a yellow calibration
rack. The frequency of calibration is every 30 days. Calibration of
this glucose procedure is accomplished by use of the Beckman Coulter
Chemistry Calibrator (Cat No. DR0070).
Calibration Procedure:
----------------------
Recalibration of this test is required when any of these conditions
exist:
1.
A reagent lot number has changed or there is an observed shift in
control values.
2.
Major preventative maintenance was performed on the analyzer.
3. A critical part was replaced.
QUALITY CONTROL:
================
During operation of the Beckman Coulter AU analyzer at least two
levels of an appropriate quality control material should be tested a
minimum of once a day. In addition, controls should be performed after
calibration, with each new lot of reagents, and after specific
maintenance or troubleshooting steps described in the appropriate AU
User’s Guide. Quality control testing should be performed in
accordance with regulatory requirements and each laboratory’s standard
procedure.
Location of controls used at this laboratory.
ANALYZER PARAMETERS:
====================
A complete list of test parameters and operating procedures can be
found in the appropriate User’s Guide and at www.beckmancoulter.com.
CALCULATIONS:
=============
For SI units (mmol/L), multiply the results by 0.0555.
REPORTING RESULTS:
==================
Reference Ranges:
-----------------
Serum6: Adult: 70 - 105 mg/dL
Newborn: 21 - 58 mg/dL
Urine: There should be no detectable glucose in urine.
Cerebrospinal fluid7: Child: 60 - 80 mg/dL
Adult: 40 - 70 mg/dL
Expected values may vary with age, sex, diet and geographical
location. Each laboratory should determine its own expected values as
dictated by good laboratory practice.
Expected reference ranges in this laboratory:
Procedures for Abnormal Results:
--------------------------------
Abnormal results are flagged by the listed analyzers according to the
normal values entered by the user into the instrument parameters.
Reporting Format:
-----------------
Results are automatically printed for each sample in mg/dL at 37°C.
Additional reporting information as designated by this laboratory:
LIMITATIONS:
============
The Beckman Coulter AU System Glucose procedure is linear from 10 -
800 mg/dL for serum determinations; 10 - 700 mg/dL for urine
determinations. Samples exceeding the upper limit of linearity should
be diluted and repeated. The sample may be diluted, repeated and
multiplied by the dilution factor automatically utilizing the AUTO
REPEAT RUN.
Interfering Substances:
-----------------------
Results of studies8 show that the following substances interfere with
this glucose procedure.
The criteria for no significant interference is recovery within 10% of
the initial value.
Bilirubin:
No significant interference up to 40 mg/dL Bilirubin
Hemolysis:
No significant interference up to 500 mg/dL Hemolysate
Lipemia:
No significant interference up to 700 mg/dL Intralipid*
* Intralipid, manufactured by KabiVitrium Inc., is a 20% IV fat
emulsion used to emulate extremely turbid samples.
The information presented is based on results from Beckman Coulter
studies and is current at the date of publication. Beckman Coulter
Inc., makes no representation about the completeness or accuracy of
results generated by future studies. For further information on
interfering substances, refer to Young9 for a compilation of reported
interferences with this test.
Laboratory specific procedure notes:
REFERENCES:
===========
1. Stein, M.W., Clinical Methods of Enzymatic Analysis, Academic
Press, 117, 1965.
2. Kaplan, L.A. and Pesce, A.J., Clinical Chemistry Theory, Analysis
and Correlation, C.V. Mosby., St. Louis, 1989.
3. Neely, W.E., Clin Chem 18: 509, 1972.
4. Keller, D.M., Clin Chem 11: 471, 1965.
5. Yee, H.Y., Clin Chem 17: 648, 1971.
6. Bondar, R.J.L. and Mead, D.C., Clin Chem, 20: 586, 1974.
7 Tietz, N.W.(ed), Clincial Guide to Laboratory Tests, Second Edition,
W.B. Saunders, 1990.
8. CLSI/NCCLS, Interference Testing in Clinical Chemistry, EP7-A,
2002.
9. Young, D.S., Effects of Drugs on Clinical Laboratory Tests, AACC
Press, Fifth Edition, 2000.
10. CLSI/NCCLS Evaluation Protocol EP5-A, 1999.
11. Data on file for specific AU analyzers.
© Beckman Coulter, Inc. 2011 Page 15 of 15
All printed copies are considered to be copies of the electronic
original. Rev #2, July 2011
 MODELO DE DECLARACIÓN RESPONSABLE DDª CON DNI
MODELO DE DECLARACIÓN RESPONSABLE DDª CON DNI 4 CARTA (26) A LOS DISCÍPULOS (17III2002) DESDE EL
4 CARTA (26) A LOS DISCÍPULOS (17III2002) DESDE EL 4(4) MERITFÖRTECKNING LÄKARTJÄNST VID LUS IFYLLES ELEKTRONISKT AV DEN
4(4) MERITFÖRTECKNING LÄKARTJÄNST VID LUS IFYLLES ELEKTRONISKT AV DEN R ADTAGESTOURENPROGRAMM 2019 2 BEI UNSICHERER WETTERLAGE ODER
R ADTAGESTOURENPROGRAMM 2019 2 BEI UNSICHERER WETTERLAGE ODER BASES DE LA CONVOCATORIA DE BECAS DEPORTIVAS DEL CENTRO
BASES DE LA CONVOCATORIA DE BECAS DEPORTIVAS DEL CENTRO ACTIVACIÒN DEL FACTOR NUCLEAR KB EN LAS ETAPAS INICIALES
ACTIVACIÒN DEL FACTOR NUCLEAR KB EN LAS ETAPAS INICIALES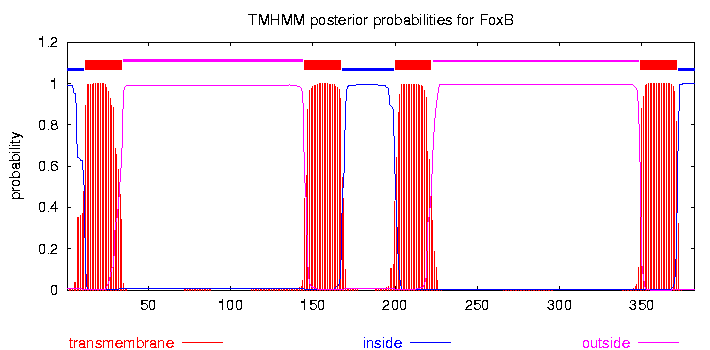 CHAPTER FIVE FOXB OF PSEUDOMONAS AERUGINOSA PAO1 PROTEIN EXPRESSION
CHAPTER FIVE FOXB OF PSEUDOMONAS AERUGINOSA PAO1 PROTEIN EXPRESSION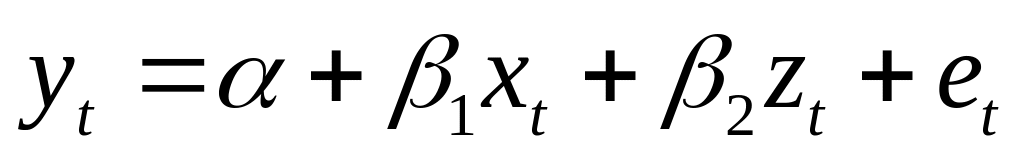 GENERALIZED LEAST SQUARES NOTES ASSUME A MODEL (1) WHERE
GENERALIZED LEAST SQUARES NOTES ASSUME A MODEL (1) WHERE ČASOVNICA IN PROGRAM TEKMOVANJA DO 900 PARKIRIŠČE NA KOSOVELOVI
ČASOVNICA IN PROGRAM TEKMOVANJA DO 900 PARKIRIŠČE NA KOSOVELOVI TITLE ASSOCIATION OF CARIES ACTIVITY WITH THE COMPOSITION OF
TITLE ASSOCIATION OF CARIES ACTIVITY WITH THE COMPOSITION OF VYSOKÁ ŠKOLA OBCHODNÍ A HOTELOVÁ PROGRAM VŠOH V BUDOVĚ
VYSOKÁ ŠKOLA OBCHODNÍ A HOTELOVÁ PROGRAM VŠOH V BUDOVĚ