drug information sheet(kusuri-no-shiori) internal revised: 11/2016 the information on this sheet is based on approvals granted by the
Drug Information Sheet("Kusuri-no-Shiori")
Internal
Revised: 11/2016
The information on this sheet is based on approvals granted by the
Japanese regulatory authority. Approval details may vary by country.
Medicines have adverse reactions (risks) as well as efficacies
(benefits). It is important to minimize adverse reactions and maximize
efficacy. To obtain a better therapeutic response, patients should
understand their medication and cooperate with the treatment.
Brand name:ZOLMITRIPTAN OD TABLETS 2.5mg "TOWA"
Active ingredient:Zolmitriptan
Dosage form:yellowish white tablet, diameter: 7.0 mm, thickness: 3.3
mm
Print on wrapping:ゾルミトリプタンOD2.5mg「トーワ」,2.5mg,Zolmitriptan OD 2.5,口腔内崩壊錠,片頭痛の薬
Effects of this medicine
This medicine is used to treat migraine, acting as a 5-HT1B/1D
receptor agonist to reduce swelling of blood vessels around the brain.
It controls/treats various symptoms (nausea, vomiting, sensitivity to
light and sound) including headache in the event of migraine attack.
However, it does not reduce the frequency of symptoms.
Before using this medicine, be sure to tell your doctor and pharmacist
・If you ever experienced any allergic reaction (itch, rash etc.) to
any medicine.
If you have heart disease or a history of heart disease.
If you have a history of cerebrovascular disease or transient ischemic
attack (TIA).
If you have a disorder in the peripheral blood vessels or
hypertension.
・If you are pregnant or breastfeeding.
・If you are taking any other medicinal products. (Some medicines may
interact to enhance or diminish medicinal effects. Beware of
over-the-counter medicines and dietary supplements as well as other
prescription medicines.)
Dosing schedule (How to take this medicine)
・Your dosing schedule prescribed by your doctor is(( to be written by
a healthcare professional))
・In general, for adults, take one tablet (2.5mg of active ingredient)
when migraine pain begins. If the effect is insufficient with one
tablet, subsequent attacks can be treated with 2 tablets (5mg of
active ingredient) but wait at least 2 hours from the first dose. Do
not take more than 4 tablets (10mg of active ingredient) in one day.
Strictly follow the dosing instructions given by your doctor.
・This medicine should be taken only after the onset of migraine. DO
NOT take the medicine when you have no pain in order to prevent
migraine.
・If this medicine does not work at all, do not take another dose.
Check with your doctor.
・Do not take this medicine to treat a headache that feels different
from your usual migraine attacks (e.g. abnormal pain, symptoms,
course).
・This medicine is orally disintegrating tablet. Take this medicine in
either of the following ways:
(1) Take this medicine with water or warm water as you do an ordinary
tablet.
(2) Moisten the tablet with saliva on your tongue and crush it lightly
with your tongue, then swallow it with saliva. However, never take the
tablet without water when you are in a lying position.
・If you accidentally take more than your prescribed dose, consult with
your doctor or pharmacist.
・Do not stop taking this medicine unless your doctor instructs you to
do so.
Precautions while taking this medicine
・Avoid driving a car or operate dangerous machinery until your
migraine is gone, since this medicine may cause drowsiness in the
recovering phase of migraine.
Possible adverse reactions to this medicine
The most commonly reported adverse reactions include nausea,
derangement of sensory, somnolence (drowsiness), worsened migraine,
chest constriction, malaise, palpitation, dizziness, abnormal
sensation, hypersensitivity (urticaria/angioedema). If any of these
symptoms occur, consult with your doctor or pharmacist.
The symptoms described below are rarely seen as initial symptoms of
the adverse reactions indicated in brackets. If any of these symptoms
occur, stop taking this medicine and see your doctor immediately.
・respiratory distress, hives, swelling of eye/mouth area [anaphylactic
shock, anaphylaxis]
・irregular pulse [arrhythmia]
・acutely pressured feeling the chest, cold sweat, anginal pain
[ischemic heart disease symptoms]
・palpitation and fast pulse with discomfort in chest (over 100/min.)
[tachycardia]
・consciousness disorder, convulsion [epileptiform attack]
The above symptoms do not describe all the adverse reactions to this
medicine. Consult with your doctor or pharmacist if you notice any
symptoms of concern other than those listed above.
Storage conditions and other information
・Keep out of reach of children. Store away from direct sunlight, heat
and moisture.
・Discard the remainder. Do not store them. Ask the pharmacy or medical
institution how to discard.
For healthcare professional use only / /
For further information, talk to your doctor or pharmacist.
2 / 2
 LA CHASSE ET LE DROIT PRÉAMBULE VOUS TROUVEREZ DANS
LA CHASSE ET LE DROIT PRÉAMBULE VOUS TROUVEREZ DANS GYVENIMO APRAŠYMAS MARIJA CHLEBOPAŠEVAITĖ M2399 W WWFACEBOOKCOMMARIJA CHLEBOPASEŠEVAITĖ LYTIS
GYVENIMO APRAŠYMAS MARIJA CHLEBOPAŠEVAITĖ M2399 W WWFACEBOOKCOMMARIJA CHLEBOPASEŠEVAITĖ LYTIS BOSNA I HERCEGOVINA FEDERACIJA FEDERACIJA BOSNE I HERCEGOVINE SREDNJOBOSANSKI
BOSNA I HERCEGOVINA FEDERACIJA FEDERACIJA BOSNE I HERCEGOVINE SREDNJOBOSANSKI RENJA BPBD TAHUN 2017 BAB I PENDAHULUAN I1 LATAR
RENJA BPBD TAHUN 2017 BAB I PENDAHULUAN I1 LATAR ACTIVITY REPORTING PROGRAMME 12 WEEK MATERNAL ASSESSMENT BREASTFEEDING
ACTIVITY REPORTING PROGRAMME 12 WEEK MATERNAL ASSESSMENT BREASTFEEDING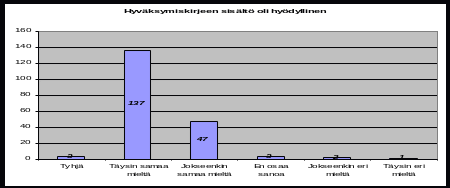 3 RAPORTTI OPINTONSA SYKSYLLÄ 2007 KÄYTTÄYTYMISTIETEELLISESSÄ TIEDEKUNNASSA ALOITTANEIDEN UUSIEN
3 RAPORTTI OPINTONSA SYKSYLLÄ 2007 KÄYTTÄYTYMISTIETEELLISESSÄ TIEDEKUNNASSA ALOITTANEIDEN UUSIEN COLÉGIO PLÍNIO LEITE REDAÇÃO – 2º PERÍODO2018 8º ANO
COLÉGIO PLÍNIO LEITE REDAÇÃO – 2º PERÍODO2018 8º ANO