ap chemistry key_chapt. 9 study guide 1. the geometry of the sf4 molecule is a. tetrahedral. b. trigonal pyramidal. c. trigonal plana
AP Chemistry
KEY_Chapt. 9 Study Guide
1. The geometry of the SF4 molecule is
A. tetrahedral. B. trigonal pyramidal. C. trigonal planar.
D. square planar. E. distorted tetrahedron (see-saw).
Answer: E
2. According to VSEPR theory, the geometry of the PH3 molecule is best
described as
A. linear. B. trigonal planar. C. tetrahedral. D. bent.
E. trigonal pyramidal.
Answer: E
3. According to the VSEPR theory, the molecular geometry of the
carbonate ion, CO32 –, is
A. square planar. B.tetrahedral. C. pyramidal. D. trigonal planar.
E. octahedral.
Answer: D
4. According to VSEPR theory, which one of the following molecules
should have a bent
shape?
A. Cl2O B. CO2 C. HCN D. CCl4 E. none of them
Answer: A
5. According to VSEPR theory, which one of the following molecules has
tetrahedral geometry?
A. NH3 B. CCl4 C. CO2 D. SF4 E. PCl5
Answer: B
6. The F –Cl –F bond angles in ClF3 are expected to be approximately
A. 90 only.
B. 109.5 only.
C. 120 only.
D. 180 only.
E. 90 and 120.
Answer: E
7. According to the VSEPR theory, the actual F –As –F bond angles in
the AsF4 ion are predicted to be
A. 109.5
B. 90 and 120
C. 180
D. < 109.5
E. < 90 and < 120
Answer: E
8. Is BeCl2 polar or nonpolar?
Answer: nonpolar
9. Is CH3Cl a polar or nonpolar molecule?
Answer: polar
10. Predict the geometry and polarity of the CS2 molecule.
A. linear, polar B. linear, nonpolar C. tetrahedral, nonpolar
D. bent, nonpolar E. bent, polar
Answer: B
11. Indicate the type of hybrid orbitals used by the central atom in
CCl4.
A. sp B. sp2 C. sp3 D. sp3d E. sp3d2
Answer: C
12. What is the hybridization of As in the AsF4 ion?
A. sp B. sp2 C. sp3 D. sp3d E. sp3d2
Answer: D
13. What is the hybridization of the As atom in the AsF5 molecule?
A. sp B. sp2 C. sp3 D. sp3d E. sp3d2
Answer: D
14. Which of the following molecules have the same geometries?
A. NH2 and H2O
B. NH2 and BeH2
C. H2O and BeH2
D. NH2, H2O, and BeH2
Answer: A
15. The number of pi bonds in the molecule below is
H H
| |
HCCCCCCH
| |
H H
A. 2 B. 4 C. 6 D. 10 E. 15
Answer: B
16. Consider the species N2, N2, and N2+. Which of these species will
be paramagnetic, according to MO theory?
A. N2 and N2 B. N2+ and N2 C. N2+ and N2 D. Only N2
E. None are paramagnetic
Answer: C
17. In which of the following would the bonding be strengthened with
the addition of an electron to form the negative molecular ion?
A. N2 B. O2 C. F2 D. All of these E. None of these
Answer: E
18. Which of the following is not true of molecular orbitals?
A. The number of molecular orbitals formed is always equal to the
number of atomic orbitals combined.
B. A molecular orbital can accommodate up to two electrons.
C. When electrons are added to orbitals of the same energy, the most
stable arrangement is predicted by Hund’s rule.
D. Low-energy molecular orbitals fill before high-energy molecular
orbitals fill.
E. For any substance, the number of electrons in molecular orbitals is
equal to the sum of all the valence electrons on the bonding atoms.
Answer: E
19. The electrons in the delocalized molecular orbitals of benzene (C6H6)
A. are confined between two adjacent bonding atoms.
B. are free to move around the six-membered ring.
C. form the electron pairs in the C–H bonds of the compound.
D. are unevenly distributed through the molecule.
E. are responsible for the fact that the bonds between three pairs of
carbon atoms in the ring are longer and stronger than the bonds
between the other three pairs of carbon atoms.
Answer: B
20. True or False: The hybridization of N in the NH3 molecule is sp3.
Answer: T
21. Using periodic trends, arrange the following molecules in order of
increasing dipole
moment: NH3, PH3, AsH3
Answer: AsH3 < PH3 < NH3
22. Explain why CH4 is nonpolar, but CH2Cl2 is polar.
Answer: Both molecules have a tetrahedral shape, which is symmetrical.
In CH4, the individual dipole moments are equal in magnitude, and thus
cancel each other out. In CH2Cl2, the C-Cl bonds have a larger dipole
moment than the C-H bonds and so do not cancel out.
 NR 7752 28062016 SE APROBĂ DIRECTOR GENERAL DR ING
NR 7752 28062016 SE APROBĂ DIRECTOR GENERAL DR ING PLANTAS ALZADOS Y SECCIONESALGO MÁS QUE UNAS PROYECCIONES ORTOGONALES
PLANTAS ALZADOS Y SECCIONESALGO MÁS QUE UNAS PROYECCIONES ORTOGONALES SPECIFICATION TEXT PRODUCT MELAMINE FACED PANELS DATE 251121 REFERENCE
SPECIFICATION TEXT PRODUCT MELAMINE FACED PANELS DATE 251121 REFERENCE F SADDLEBACK VALLEY UNIFIED SCHOOL DISTRICT ETHS
F SADDLEBACK VALLEY UNIFIED SCHOOL DISTRICT ETHS  OCTOBER 2014 EMFECBC GUIDELINES ON THE INDEPENDENCE OF VALUERS
OCTOBER 2014 EMFECBC GUIDELINES ON THE INDEPENDENCE OF VALUERS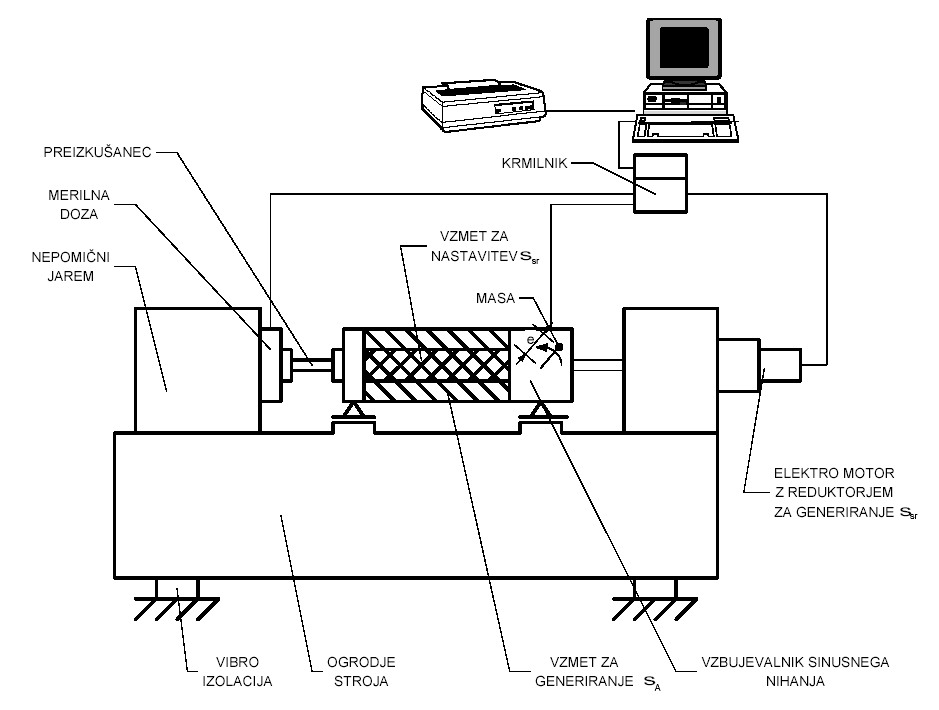 VAJA 1 DINAMIČNA TRDNOST VAJA 1 DINAMIČNA TRDNOST KAZALO
VAJA 1 DINAMIČNA TRDNOST VAJA 1 DINAMIČNA TRDNOST KAZALO NOM DATA ÀREA DE MATEMÀTIQUES PROVA 1 AVA3 20072008
NOM DATA ÀREA DE MATEMÀTIQUES PROVA 1 AVA3 20072008