title of the article journal name, 2019, vol. 0, no. 0 0 article type title: (the title of the article should be precise and brief and must
Title of the Article Journal Name, 2019, Vol. 0, No. 0 0
ARTICLE TYPE
Title: (The Title of the Article should be Precise and Brief and Must
Not be More Than 120 Characters. Authors should avoid the Use of
Non-Standard Abbreviations. The Title Must be Written in Title Case
Except for articles, conjunctions and prepositions.)
Principle Authora, Corresponding authorb*, Co-author, Co-authora and
Co-authorb
aDepartment Name, Faculty Name, University Name, City, Country; bDepartment
Name, Faculty Name, University Name, City, Country
Abstract: The abstract of an article should be clear, concise and
accurate summary, having no more than 250 words, and including the
explicit sub-headings (as in-line or run-in headings in bold). Use of
abbreviations should be avoided and the references should not be cited
in the abstract. Ideally, each abstract should include the following
sub-headings, but these may vary according to requirements of the
article.
*
Background
*
Objective
*
Method
*
Results
*
Conclusion
A R T I C L E H I S T O R Y
Received:
Revised:
Accepted:
DOI:
Keywords: Provide 6 to 8 keywords.
1. INTRODUCTION
The Introduction section should include the background and aims of the
research in a comprehensive manner.
1.1. Section Headings
Section headings should be numbered sequentially, left aligned and
have the first letter capitalized, starting with the introduction.
Sub-section headings however, should be in lower-case and italicized
with their initials capitalized. They should be numbered as 1.1, 1.2,
etc.
1.2. Text Organization
Please provide soft copies of all the materials (main text in MS Word
or Tex/LaTeX), figures/illustrations in TIFF, PDF or JPEG, and
chemical structures drawn in ChemDraw (CDX)/ISISDraw (TGF) as separate
files, while a PDF version of the entire manuscript must also be
included, embedded with all the figures/illustrations/tables/chemical
structures etc. It is advisable that the document files related to a
manuscript submission should always have the name of
*Address correspondence to this author at the Department of xxxy,
Faculty of xxx, xxx University, P.O. Box: 0000-000, City, Country;
Tel/Fax: ++0-000-000-0000, +0-000-000-0000; E-mails:
[email protected]
the corresponding author as part of the file name, i.e., “Cilli MS
text.doc”, “Cilli MS Figure 1” etc.
It is imperative that before submission, authors should carefully
proofread the files for special characters, mathematical symbols,
Greek letters, equations, tables,
references and images, to ensure that they appear in proper format.
The main text should begin on a separate page and should be divided
into title page, abstract and the main text. The text may be
subdivided further according to the areas to be discussed, which
should be followed by the Acknowledgements and Reference sections. For
Research papers, the manuscript should begin with the title page and
abstract followed by the main text, which must be structured into
separate sections as Introduction, Materials and Methods, Conclusion,
Conflict of Interest, Acknowledgements and References. The Review
Article should mention any previous important recent and old reviews
in the field and contain a comprehensive discussion starting with the
general background of the field. It should then go on to discuss the
salient features of recent developments. The authors should avoid
presenting material which has already been published in a previous
review. The authors are advised to present and discuss their
observations in brief.
References, figures, tables, chemical structures etc. should be
referred to in the text at the appropriate place where they have been
first discussed. Figure legends/captions should also be provided.
1.3. Figures/Illustrations
All authors must strictly follow the guidelines below for preparing
illustrations for publication in Current Topics in Medicinal
Chemistry. If the figures are found to be sub-standard, then the
manuscripts will be rejected.
The authors are expected to submit good quality figure(s) in PDF, PPT,
MS Word, TIFF or JPEG versions, which, if required, should be improved
yourself or by professional graphic designers of your organization/
country. You may even consider approaching our contracted service
providers Eureka Science for Graphics Enhancement Services.
The Graphics Designing team at Eureka Science can assist in improving
the quality of your images at affordable rates. Eureka Science has
contracted special rates with us of US $125 for the improvement of up
to five figures, with any additional figures being charged at US $20
each.
The quality of Graphic Enhancement Services offered by Eureka Science
can be viewed at http://www.eureka-science.com/images/Binder1.pdf,
along with valuable feedback on their services at
http://www.eureka-science.com/testimonials.php. You may contact Eureka
Science at [email protected]
Note: Availing Graphics Enhancement Services do not guarantee
acceptance of the manuscript for publication. The final
acceptance/decision on the manuscript is taken by the EiC.
1.3.1 Guideline for Figures/Illustrations
Illustrations must be provided according to the following guideline:
Illustrations should be embedded in the text file, and must be
numbered consecutively in the order of their appearance. Each figure
should include only a single illustration which should be cropped to
minimize the amount of space occupied by the illustration.
If a figure is in separate parts, all parts of the figure must be
provided in a single composite illustration file.
Photographs should be provided with a scale bar if appropriate, as
well as high-resolution component files.
1.3.2. Scaling/Resolution
Line Art image type is normally an image based on lines and text. It
does not contain tonal or shaded areas. The preferred file format
should be TIFF or EPS, with the color mode being Monochrome 1-bit or
RGB, in a resolution of 900-1200 dpi.
Halftone image type is a continuous tone photograph containing no
text. It should have the preferred file format TIFF, with color mode
being RGB or Grayscale, in a resolution of 300 dpi.
Combination image type is an image containing halftone , text or line
art elements. It should have the preferred file format TIFF, with
color mode being RGB or Grayscale, in a resolution of 500-900 dpi.
1.3.3. Formats
Illustrations may be submitted in the following file formats
*
Illustrator
*
EPS (preferred format for diagrams)
*
PDF (also especially suitable for diagrams)
*
PNG (preferred format for photos or images)
*
Microsoft Word (version 5 and above; figures must be a single
page)
*
PowerPoint (figures must be a single page)
*
TIFF
*
JPEG (conversion should be done using the original file)
*
BMP
*
CDX (ChemDraw)
*
TGF (ISISDraw)
Bentham Science Publishers does not process figures submitted in GIF
format.
For TIFF or EPS figures with considerably large file size restricting
the file size in online submissions is advisable. Authors may
therefore convert to JPEG format before submission as this results in
significantly reduced file size and upload time, while retaining
acceptable quality. JPEG is a ‘lossy’ format. However, in order to
maintain acceptable image quality, it is recommended that JPEG files
are saved at High or Maximum quality.
Zipit or Stuffit tools should not be used to compress files prior to
submission as the resulting compression through these tools is always
negligible.
Please refrain from supplying
1.
Graphics embedded in word processor (spreadsheet, presentation)
document.
2.
Optimized files optimized for screen use (like GIF, BMP, PICT,
WPG) because of the low resolution.
3.
Files with too low a resolution.
4.
Graphics that are disproportionately large for the content.
Technical requirements for graphic/ figure submissions.
1.3.4 Requirement
Width = 8.5 inches (In-between the required size)
Height = 11 inches (In-between the required size)
Pixels/Inches = 300 (minimum dpi)
All figures should be in vector scale (except half tone, photograph.)
1.3.5. Image Conversion Tools
There are many software packages, many of them freeware or shareware,
capable of converting to and from different graphics formats,
including PNG.
General tools for image conversion include Graphic Converter on the
Macintosh, Paint Shop Pro, for Windows, and ImageMagick, available on
Macintosh, Windows and UNIX platforms.
Bitmap images (e.g. screenshots) should not be converted to EPS as
they result in a much larger file size than the equivalent JPEG, TIFF,
PNG or BMP, and poor quality. EPS should only be used for images
produced by vector-drawing applications such as Adobe Illustrator or
CorelDraw. Most vector-drawing applications can be saved in, or
exported as, EPS format. If the images were originally prepared in an
Office application, such as Word or PowerPoint, original Office files
should be directly uploaded to the site, instead of being converted to
JPEG or another format of low quality.
1.3.6. Color Figures/Illustrations
*
The cost for each individual page of color
figures/plates/illustrations is US$ 1045.
*
Color figures should be supplied in CMYK and not in RGB colors.
Colour figures should be supplied in CMYK not RGB colors.
1.3.7. Chemical Structures
Chemical structures must be prepared in ChemDraw (CDX file) and
provided as separate file.
1.4. Symbols and Units
Greek symbols and special characters often undergo formatting changes
and get corrupted or lost during preparation of a manuscript for
publication. To ensure that all special characters used are embedded
in the text, these special characters should be inserted as a symbol
but should not be a result of any format styling (Symbol font face)
otherwise they will be lost during the conversion to PDF/XML.
Authors are encouraged to consult reporting guidelines. These
guidelines provide a set of recommendations comprising a list of items
relevant to their specific research design.
Only ISO symbols, written in italic, should be used for the various
parameters. All kinds of measurements should be reported only in
International System of Units (SI). SI units should always be written
in Roman and separated from the numerical value by a space (whatever
the language).
The µ in µg or µm should be in Roman. The symbol for litre is L and
that for minute is min. For temperature, please use only one of °C, °F
or K in the entire manuscript. As the Angström (1Å = 10-10m) is not an
SI unit, it should be replaced by the nanometre (1nm = 10-9 m) or by
the picometer (1pm = 10-12 m): 1Å = 0.1nm = 100 pm. Multiple units
should be written with negative superscripts (for example, 25mgµL-1
µs-1). The list of notations should appear just before the first
paragraph of full text.
A list of symbols and units should be provided if used extensively
throughout the text.
1.5. Tables
*
Data Tables should be submitted in Microsoft Word table format.
*
Each table should include a title/caption being explanatory in
itself with respect to the details discussed in the table.
Detailed legends may then follow.
*
Table number in bold font i.e. Table 1, should follow a title. The
title should be in small case with the first letter in caps. A
full stop should be placed at the end of the title.
*
Tables should be embedded in the text exactly according to their
appropriate placement in the submitted manuscript.
*
Columns and rows of data should be made visibly distinct by
ensuring that the borders of each cell are displayed as black
lines.
*
Tables should be numbered in Arabic numerals sequentially in order
of their citation in the body of the text.
*
If a reference is cited in both the table and text, please insert
a lettered footnote in the table to refer to the numbered
reference in the text.
*
Tabular data provided as additional files can be submitted as an
MS Excel spreadsheet.
1.6. Construction of References
All references should be numbered sequentially [in square brackets] in
the text and listed in the same numerical order in the reference
section. The reference numbers must be finalized and the bibliography
must be fully formatted before submission.
Sample references are provided at the end of this template in the
reference section. Correct reference format and list must be provided
in the article.
2. MATERIALS AND METHOD (FOR RESEARCH ARticles only)
This section provides details of the methodology used along with
information on any previous efforts with corresponding references. Any
details for further modifications and research should be included.
EXPERIMENTAL (FOR RESEARCH ARticles only)
Repeated information should not be reported in the text of an article.
A calculation section must include experimental data, facts and
practical development from a theoretical perspective.
3. RESULTS (FOR RESEARCH ARticles only)
The Results and discussions may be presented individually or combined
in a single section with short and informative headings.
4. DISCUSSION
This should explore the significance of the results of the work,
present a reproducible procedure and emphasis the importance of the
article in the light of recent developments in the field. Extensive
citations and discussion of published literature should be avoided.
The Results and Discussion may be presented together under one heading
of “Results and Discussion”. Alternatively, they may be presented
under two separate sections (“Results” section and “Discussion”
Sections). Short sub- headings may be added in each section if
required.
CONCLUSION
A small paragraph summarizing the contents of the article, presenting
the final outcome of the research or proposing further study on the
subject, may be given at the end of the article under the Conclusion
section.
LIST OF ABBREVIATIONS
If abbreviations are used in the text either they should be defined in
the text where first used, or a list of abbreviations can be provided.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All clinical investigations must be conducted according to the
Declaration of Helsinki principles. For all manuscripts reporting data
from studies involving human participants, formal review and approval
by an appropriate institutional review board or ethics committee is
required.
For research involving animals, the authors should indicate whether
the procedures followed were in accordance with the standards set
forth in the eighth edition of Guide for the Care and Use of
Laboratory Animals (grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals_prepub.pdf
published by the National Academy of Sciences, The National Academies
Press, Washington, D.C.).
HUMAN AND ANIMAL RIGHTS
Research work on animals should be carried out in accordance with the
NC3Rs ARRIVE Guidelines. For In Vivo Experiments, visit
https://www.nc3rs.org.uk/arrive-guidelines
Authors must clearly state the name of the approval committee,
highlighting that legal and ethical approval was obtained prior to
initiation of the research work carried out on animals, and that the
experiments were performed in accordance with the relevant guidelines
and regulations stated below.
*
US authors should cite compliance with the US National Research
Council's "Guide for the Care and Use of Laboratory Animals"
*
The US Public Health Service's "Policy on Humane Care and Use of
Laboratory Animals" and "Guide for the Care and Use of Laboratory
Animals"
*
UK authors should conform to UK legislation under the Animals
(Scientific Procedures) Act 1986 Amendment Regulations (SI
2012/3039).
*
European authors outside the UK should conform to Directive
2010/63/EU.
*
Research in animals must adhere to ethical guidelines of The Basel
Declaration and the International Council for Laboratory Animal
Science (ICLAS) has also published ethical guidelines.
*
The manuscript must clearly include a declaration of compliance
with relevant guidelines (e.g. the revised Animals (Scientific
Procedures) Act 1986 in the UK and Directive 2010/63/EU in Europe)
and/or relevant permissions or licences obtained by the IUCN
Policy Statement on Research Involving Species at Risk of
Extinction and the Convention on the Trade in Endangered Species
of Wild Fauna and Flora.
RESEARCH INVOLVING PLANTS
All experimental research on plants (either cultivated or wild), must
comply with international guidelines. The manuscript must clearly
include a declaration of compliance of field studies with relevant
guidelines and/or relevant permissions or licences obtained by the
IUCN Policy Statement on Research Involving Species at Risk of
Extinction and the Convention on the Trade in Endangered Species of
Wild Fauna and Flora.
CONSENT FOR PUBLICATION
If the manuscript has an individuals’ data, such as personal detail,
audio-video material etc., consent should be obtained from that
individual. In case of children, consent should be obtained from the
parent or the legal guardian.
A specific declaration of such approval and consent-to-disclose form
must be made in the copyright letter and in a stand-alone paragraph at
the end of the article especially in the case of human studies where
inclusion of a statement regarding obtaining the written informed
consent from each subject or subject's guardian is a must. The
original should be retained by the guarantor or corresponding author.
Editors may request to provide the original forms by fax or email.
All such case reports should be followed by a proper consent prior to
publishing.
AVAILABILITY OF DATA AND MATERIALS
The source of data and materials should be mentioned in the
manuscript, in support of the findings. If the data source is not
revealed, the authors need to clearly state the reasons. Authors who
do not wish to share their data should clearly state that the data
will not be shared, and give the reasons.
The statement relating to the data should be presented in the
following format under a separate ‘Availability of Data and Materials’
section in the manuscript:
"The data supporting the findings of the article is available in the
[repository name] at [URL], reference number [reference number]”.
FUNDING
Authors must list the source(s) of funding for the study. This should
be done for each author.
CONFLICT OF INTEREST
Financial contributions and any potential conflict of interest must be
clearly acknowledged under the heading ‘Conflict of Interest’. Authors
must list the source(s) of funding for the study. This should be done
for each author.
ACKNOWLEDGEMENTS
All individuals listed as authors must have contributed substantially
to the design, performance, analysis, or reporting of the work and are
required to indicate their specific contribution. Anyone
(individual/company/institution) who has substantially contributed to
the study for important intellectual content, or who was involved in
the article’s drafting the manuscript or revising must also be
acknowledged.
Guest or honorary authorship based solely on position (e.g. research
supervisor, departmental head) is discouraged.
The specific requirements for authorship have been defined by the
International Committee of Medical Journal Editors (ICMJE;
www.icmje.org). Examples of authors' contributions are: 'designed
research/study', 'performed research/study', 'contributed important
reagents', 'collected data', 'analyzed data', 'wrote paper' etc. This
information must be included in the submitted manuscript as a separate
paragraph under the heading ‘Acknowledgements’. The corresponding
author is responsible for obtaining permission from all co-authors for
the submission of any version of the manuscript and for any changes in
the authorship.
SUPPORTIVE/SUPPLEMENTARY MATERIAL
Supportive/Supplementary material intended for publication must be
numbered and referred to in the manuscript but should not be a part of
the submitted paper. In-text citations as well as a section with the
heading "Supportive/Supplementary Material" before the "References"
section should be provided. All Supportive/Supplementary Material must
be listed and a brief caption line for each file describing its
contents should be included.
REFERENCES
References must be listed in the ACS style only. All references should
be numbered sequentially [in square brackets] in the text and listed
in the same numerical order in the reference section. The reference
numbers must be finalized and the bibliography must be fully formatted
before submission.
See below few examples of references listed in the ACS Style:
Journal Reference
*
[1] Bard, M.; Woods, R.A.; Bartón, D.H.; Corrie, J.E.; Widdowson,
D.A. Sterol mutants of Saccharomyces cerevisiae: chromatographic
analyses. Lipids, 1977, 12(8), 645-654.
*
[2] Zhang, W.; Brombosz, S.M.; Mendoza, J.L.; Moore, J.S. A
high-yield, one-step synthesis of o-phenylene ethynylene cyclic
trimer viaprecipitation-driven alkyne metathesis. J. Org. Chem.,
2005, 70, 10198-10201.
Book Reference
*
[3] Crabtree, R.H. The Organometallic Chemistry of the Transition
Metals, 3rd ed.; Wiley & Sons: New York, 2001.
Book Chapter Reference
[4] Wheeler, D.M.S.; Wheeler, M.M. D. Stereoselective Syntheses of
Doxorubicin and Related Compounds In: Studies in Natural Products
Chemistry; Atta-ur-Rahman, Ed.; Elsevier Science B. V: Amsterdam, 1994;
Vol. 14, pp. 3-46.
Conference Proceedings
*
[5] Jakeman, D.L.; Withers, S.G.E. In: Carbohydrate
Bioengineering: Interdisciplinary Approaches, Proceedings of the 4th
Carbohydrate Bioengineering Meeting, Stockholm, Sweden, June
10-13, 2001; Teeri, T.T.; Svensson, B.; Gilbert, H.J.; Feizi, T.,
Eds.; Royal Society of Chemistry: Cambridge, UK, 2002; pp. 3-8.
URL (WebPage)
*
[6] National Library of Medicine. Specialized Information
Services: Toxicology and Environmental Health.sis.nlm.nih.gov/Tox/ToxMain.html
(Accessed May 23, 2004).
Patent
*
[7] Hoch, J.A.; Huang, S. Screening methods for the identification
of novel antibiotics. U.S. Patent 6,043,045, March 28, 2000.
Thesis
*
[8] Mackel, H. Capturing the Spectra of Silicon Solar Cells. PhD
Thesis, The Australian National University: Canberra, December
2004.
E-citations
*
[9] Citations for articles/material published exclusively online
or in open access (free-to-view), must contain the accurate Web
addresses (URLs) at the end of the reference(s), except those
posted on an author’s Web site (unless editorially essential),
e.g.‘Reference: Available from: URL’.
Some important points to remember:
*
All references must be complete and accurate.
*
All authors must be cited and there should be no use of the phrase
et al.
*
Date of access should be provided for online citations.
*
Journal names should be abbreviated according to the Index
Medicus/MEDLINE.
*
Punctuation should be properly applied as mentioned in the
examples given above.
*
Superscript in the in-text citations and reference section should
be avoided.
*
Abstracts, unpublished data and personal communications (which can
only be included if prior permission has been obtained) should not
be given in the references section. The details may however appear
in the footnotes.
*
The authors are encouraged to use a recent version of EndNote
(version 5 and above) or Reference Manager (version 10) when
formatting their reference list, as this allows references to be
automatically extracted.
 MODULE 2 INQUIRY TABLE OF CONTENTS INTRODUCTION—1 WHERE ARE
MODULE 2 INQUIRY TABLE OF CONTENTS INTRODUCTION—1 WHERE ARE DISCAPACIDAD SEVERA Y VIDA AUTONOMA INDICE INTRODUCCION
DISCAPACIDAD SEVERA Y VIDA AUTONOMA INDICE INTRODUCCION 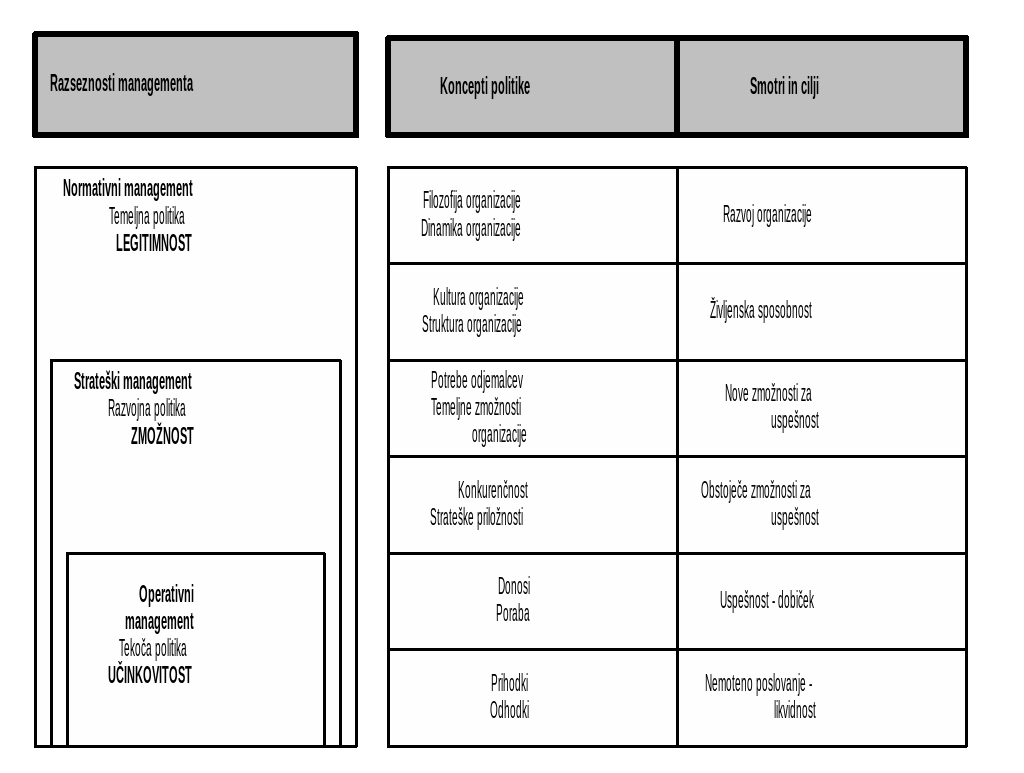 VIŠJA STROKOVNA ŠOLA 20002001 MANAGEMENT PODJETJA – PROJEKTNO VODENJE
VIŠJA STROKOVNA ŠOLA 20002001 MANAGEMENT PODJETJA – PROJEKTNO VODENJE B ORANG PERMOHONAN PERJANJIAN PEMINDAHAN BAHAN (MATERIAL TRANSFER AGREEMENT)
B ORANG PERMOHONAN PERJANJIAN PEMINDAHAN BAHAN (MATERIAL TRANSFER AGREEMENT) INFORMACIJA O IZDELKIH V OKVIRU PROJEKTA LEONARDO DA VINCI
INFORMACIJA O IZDELKIH V OKVIRU PROJEKTA LEONARDO DA VINCI ZAMBOMBAS FLAMENCAS DE JEREZ NOVIEMBRE 2009 SÁBADO 21
ZAMBOMBAS FLAMENCAS DE JEREZ NOVIEMBRE 2009 SÁBADO 21 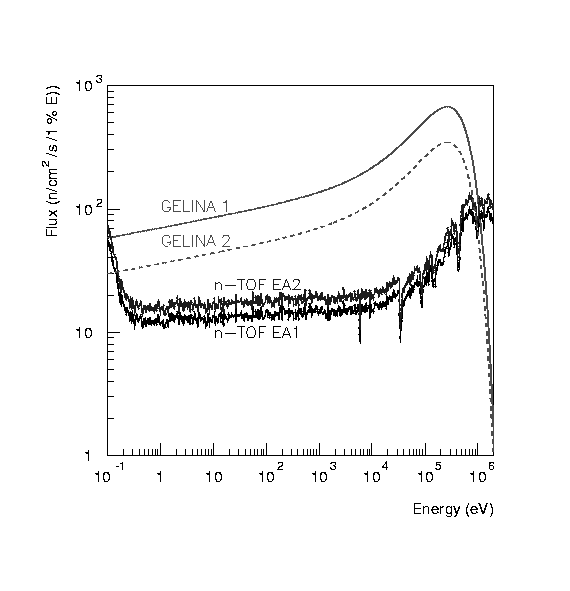 FEASIBILITY OF FISSION MEASUREMENTS AT GELINA JOSÉ BENLLIURE IGNACIO
FEASIBILITY OF FISSION MEASUREMENTS AT GELINA JOSÉ BENLLIURE IGNACIO OMPISGAEDAASU054 PÁGINA 14 S OMPISGAEDAASU054 ORIGINAL ESPAÑOL FECHA 3
OMPISGAEDAASU054 PÁGINA 14 S OMPISGAEDAASU054 ORIGINAL ESPAÑOL FECHA 3 ROLES AND RESPONSIBILITIES OF THE CUSTOMER AGENCY AND
ROLES AND RESPONSIBILITIES OF THE CUSTOMER AGENCY AND