table s1: the number of the subjects who have the complications of diabetes mellitus. basal insulin group (n = 22) premixed insuli
Table S1: The number of the subjects who have the complications of
diabetes mellitus.
Basal insulin group
(n = 22)
Premixed insulin group
(n = 21)
Total
(n = 43)
Neuropathy
(Subjects (%))
11
(50.0%)
11
(52.4%)
22
(51.2%)
Retinopathy (Subjects (%))
8
(36.4%)
10
(47.6%)
18
(41.9%)
Nephropathy
(Subjects (%))
9
(40.9%)
12
(57.1%)
21
(48.8%)
Macrovascular events (Subjects (%))
9
(40.9%)
7
(33.3%)
16
(37.2%)
Table S2: The number and ratio of the kind of the premixed insulin at
baseline.
Basal insulin gro.up
(n = 22)
Premixed insulin group
(n = 21)
Total
(n = 43)
Insulin as part 30/70 (Subjects (%))
14
(63.6%)
13
(61.9%)
27
(62.8%)
Insulin human 30/70 (Subjects (%))
1
(4.5%)
4
(19.0%)
5
(11.6%)
Insulin lispro 25/75 (Subjects (%))
5
(22.7%)
3
(14.3%)
8
(18.6%)
Insulin lispro 50/50
(Subjects (%))
2
(9.1%)
1
(4.8%)
3
(7.0%)
Table S3: The number of the subjects administered the oral
hypoglycemic agents.
Basal insulin group
(n = 22)
Premixed insulin group (n = 21)
Alpha-glucosidase inhibitor
5
3
Biguanide
3
4
Alpha-glucosidase inhibitor + Biguanide
2
3
Biguanide
+ Thiazolidinedione
1
0
Alpha-glucosidase inhibitor+ Thiazolidinedione
1
0
Alpha-glucosidase inhibitor + Biguanide+Sulfonylurea
0
1
Nothing
10
10
Table S4: The number of the subjects administered the
anti-hypertensive agents.
Basal insulin group
(n = 22)
Premixed insulin group (n = 21)
Angiotensin II receptor blocker
4
3
Angiotensin converting enzyme inhibitor
1
1
Calcium channel blocker
1
0
Diuretic
1
0
Angiotensin II receptor blocker +Calcium channel blocker
3
4
Angiotensin converting enzyme inhibitor +Calcium channel blocker
1
0
Angiotensin II receptor blocker +
Beta blocker+ Diuretic
2
1
Angiotensin II receptor blocker +
Calcium channel blocker + Alpha blocker +Diuretic
0
1
Angiotensin II receptor blocker +
Calcium channel blocker + Diuretic
0
1
Angiotensin II receptor blocker +
Calcium channel blocker + Methyldopa
0
1
Nothing
9
9
Table S5: The number of the subjects administered
anti-hypercholesterolemic agents.
Basal insulin group
(n = 22)
Premixed insulin group (n = 21)
Pitavastatin
5
5
Rosuvastatin
1
1
Simvastatin
1
0
Atorvastatin
1
0
Pravastatin
1
2
Ethyl icosapentate
1
0
Ezetimibe
1
0
Pitavastatin+
Ethyl icosapentate
0
1
Pitavastatin + Ezetimibe
0
1
Nothing
11
11
Table S6: Baseline characteristics of the subjects who performed DTSQ
survey.
Basal insulin group
(n = 17)
Premixed insulin group (n =16)
P value
Age (years)
67.2 ± 9.02
74.1 ± 7.8
0.027
Sex (male/female)
12/ 5
9 / 7
0.392
Body weight (kg)
59.4 ± 11.7
56.2 ± 10.6
0.405
Duration of diabetes (years)
15.4 ± 9.0
22.8 ± 8.8
0.025
Body mass index (kg/m2)
22.6 ± 3.7
23.2 ± 3.0
0.648
HbA1c (%)
7.97 ± 1.07
8.09 ± 1.07
0.744
Insulin dose (U/day)
21.1 ± 8.6
25.6 ± 8.5
0.135
Insulin dose (U/kg/day)
0.35 ± 0.13
0.46 ± 0.14
0.027
Systolic clinic blood pressure (mmHg)
136.3 ± 16.8
136.3 ± 22.9
0.997
Diastolic clinic blood pressure (mmHg)
75.0 ± 10.3
72.2 ± 12.6
0.447
Total cholesterol (mg/dL)
190.0 ± 49.9
186.6 ± 42.9
0.595
Triglyceride (mg/dL)
135.5 ± 84.7
123.4 ± 50.3
0.726
High-density lipoprotein cholesterol
(mg/dL)
59.4 ± 21.3
57.0 ± 12.8
0.413
Low density lipoprotein cholesterol
(mg/dL)
103.5 ± 41.1
104.9 ± 35.0
0.919
Values are expressed as means ± S.D.
Table S7: Baseline characteristics of the whole subjects in the basal
insulin group vs. the subjects who performed DTSQ survey in the basal
insulin group.
The whole of Basal insulin group
(n = 22)
The subjects who performed DTSQ
(n = 17)
P value
Age (years)
67.6 ± 8.7
67.2 ± 9.0
0.889
Sex (male/female)
14 / 8
12 / 5
0.648
Body weight (kg)
60.4 ± 12.1
59.4 ± 11.7
0.809
Duration of diabetes (years)
16.6 ± 8.6
15.4 ± 9.0
0.694
Body mass index (kg/m2)
23.2 ± 3.6
22.6 ± 3.7
0.615
HbA1c (%)
7.87 ± 0.99
7.97 ± 1.07
0.759
Insulin dose (U/day)
24.8 ± 11.1
21.1 ± 8.6
0.252
Insulin dose (U/kg/day)
0.41 ± 0.16
0.35 ± 0.13
0.239
Systolic clinic blood pressure (mmHg)
134.2 ± 16.1
136.3 ± 16.8
0.698
Diastolic clinic blood pressure (mmHg)
75.0 ± 10.3
75.4 ± 11.0
0.895
Total cholesterol (mg/dL)
190.0 ± 49.9
195.4 ± 49.9
0.742
Triglyceride (mg/dL)
135.5 ± 84.7
132.8 ± 95.2
0.928
High-density lipoprotein cholesterol
(mg/dL)
59.4 ± 21.3
62.5 ± 23.3
0.671
Low density lipoprotein cholesterol
(mg/dL)
103.5 ± 41.1
106.3 ± 41.2
0.834
Values are expressed as means ± S.D.
Table S8: Baseline characteristics of the whole subjects in the premix
insulin group vs. the subjects who performed DTSQ survey in the premix
insulin group.
The whole of Premixed insulin group
(n = 21)
The Subjects who preformed DTSQ (n =16)
P value
Age (years)
72.8 ± 10.5
74.1 ± 7.8
0.680
Sex (male/female)
12/9
9/7
0.957
Body weight (kg)
56.7 ± 9.8
56.2 ± 10.6
0.863
Duration of diabetes (years)
21.8 ± 8.8
22.8 ± 8.8
0.722
Body mass index (kg/m2)
23.3 ± 3.0
23.2 ± 3.0
0.917
HbA1c (%)
7.96 ± 1.17
8.09 ± 1.07
0.728
Insulin dose (U/day)
24.8 ± 8.8
25.6 ± 8.5
0.766
Insulin dose (U/kg/day)
0.44 ± 0.16
0.46 ± 0.14
0.757
Systolic clinic blood pressure (mmHg)
135.7 ± 20.1
136.3 ± 22.9
0.933
Diastolic clinic blood pressure (mmHg)
71.1 ± 11.3
72.2 ± 12.6
0.788
Total cholesterol (mg/dL)
188.0 ± 43.5
186.6 ± 42.9
0.927
Triglyceride (mg/dL)
124.5 ± 69.7
123.4 ± 50.3
0.960
High-density lipoprotein cholesterol
(mg/dL)
55.3 ± 12.3
57.0 ± 12.8
0.690
Low density lipoprotein cholesterol
(mg/dL)
107.7 ± 33.9
104.9 ± 35.0
0.808
Values are expressed as means ± S.D.
 ZESTAWIENIE –MEBLOWE METALOWE POZ ILOŚĆ SZTUK MODELWYKONANIE 1010 4
ZESTAWIENIE –MEBLOWE METALOWE POZ ILOŚĆ SZTUK MODELWYKONANIE 1010 4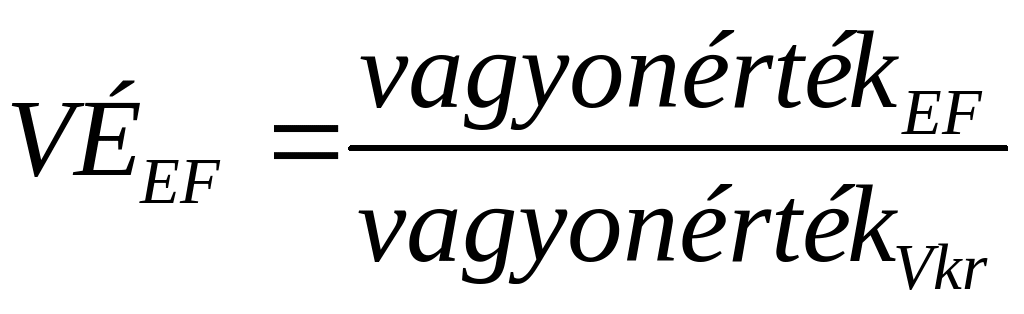 5 NAPIREND ÜGYIRATSZÁM OB4772018 E L Ő T E
5 NAPIREND ÜGYIRATSZÁM OB4772018 E L Ő T E HOMEWORK 1 YOUR TASK IS TO DESIGN A NEW
HOMEWORK 1 YOUR TASK IS TO DESIGN A NEW MODELLING OF CONJUGATE HEAT TRANSFER IN MICROELECTRONICS WITH VARIABLE
MODELLING OF CONJUGATE HEAT TRANSFER IN MICROELECTRONICS WITH VARIABLE CORONADO KIDS YOGA AT CORONADO YOGA & WELLNESS CENTER
CORONADO KIDS YOGA AT CORONADO YOGA & WELLNESS CENTER MAT251 MULTIVARIABLE CALCULUS III NAME PROF PORTER TEST
MAT251 MULTIVARIABLE CALCULUS III NAME PROF PORTER TEST