29 assessment of insulin resistance in fructose-fed rats with 125i-6-deoxy-6-iodo-d-glucose, a new tracer of glucose transport. pasc
29
Assessment of insulin resistance in fructose-fed rats with 125I-6-deoxy-6-iodo-D-glucose,
a new tracer of glucose transport.
Pascale Perret1, Lotfi Slimani1, Arnaud Briat1, Danièle Villemain1,
Serge Halimi2, Jacques Demongeot3, Daniel Fagret1, Catherine Ghezzi1
1 INSERM E0340 "Radiopharmaceutiques Biocliniques", Grenoble, France
2 Service de Diabétologie, CHU de Grenoble, France.
3 Laboratoire TIMC-IMAG -UMR 5525 CNRS, Université de Grenoble,
France.
For correspondence: P. Perret, INSERM E340 “Radiopharmaceutiques
Biocliniques”, Université de Grenoble, 38700 La Tronche, France.
Phone number: 33 (0)476 637 102
Fax number: 33 (0)476 637 142
E-mail: [email protected]
Abstract. Purpose: Insulin resistance, characterised by an
insulin-stimulated glucose transport defect, is an important feature
of the pre-diabetic state and it has been observed in numerous
pathological disorders. The purpose of this study was to assess
variations in glucose transport in rats with 125I-6-Deoxy-6-Iodo-D-glucose
(6DIG), a new tracer of glucose transport proposed as an imaging tool
to assess insulin resistance in vivo.
Methods: Two protocols were performed, a hyperinsulinaemic-euglycaemic
clamp and a normoinsulinaemic normoglycaemic protocol, in awake
control and insulin-resistant fructose-fed rats. The tracer was
injected at steady state, and activity in 11 tissues and the blood
were assessed ex vivo at several time points. A multicompartmental
mathematical model was developed to obtain fractional transfer
coefficients of 6DIG from the blood to the organs.
Results: Insulin sensitivity of fructose-fed rats, estimated by the
glucose infusion rate, was reduced by 40% compared with control rats.
At steady-state, 6DIG uptake was significantly stimulated by insulin
in insulin-sensitive tissues of control rats (basal versus insulin:
diaphragm, p<0.01; muscle, p<0.05; heart, p<0.001), whereas insulin
did not stimulate 6DIG uptake in insulin-resistant fructose-fed rats.
Moreover, in these tissues, the fractional transfer coefficients of
entrance were significantly increased with insulin in control rats
(basal vs insulin: diaphragm, p<0.001; muscle, p<0.001; heart, p<0.01)
and whereas no significant changes were observed in fructose-fed rats.
Conclusion: This study sets the stage for the future use of 6DIG as a
non-invasive means for the evaluation of insulin resistance by nuclear
imaging.
Keywords: Radiopharmaceutical, Insulin resistance, Mathematical
modelling, Nuclear medicine, Diabetes.
Introduction
The dramatic worldwide increase in the prevalence of type 2 diabetes
represents a major health problem [1]. A major feature of type 2
diabetes is that the pathology usually remains undiagnosed for as long
as 9-12 years [2], a period of time during which insulin resistance,
mostly characterised by an impairment in insulin-stimulated glucose
transport, progressively occurs in the skeletal muscle, myocardium and
adipose tissue [3-5]. Insulin resistance is the best predictor of the
future development of type 2 diabetes and probably plays a major role
in its pathogenesis [6, 7]. Several methods have been proposed for the
in vivo quantification of global insulin action through dynamic
interventions or steady-state assessment, the gold standard remaining
the hyperinsulinaemic glucose clamp [8]. However, the complexity and
length of this technique render it unsuitable for routine clinical
use. Moreover, it appears that tissue-specific, metabolite-specific
and process-specific responses can occur, which cannot be assessed
using a global measure of insulin resistance such as that provided by
the hyperinsulinemic glucose clamp technique [8].
Nuclear Medicine has the potential to provide a suitable means of
assessing regional insulin resistance in a non-invasive manner using
appropriate radiolabelled tracers. The most common approach is the
physiological modelling of dynamic positron emission tomography (PET)
imaging using 18F-2-fluoro-2-deoxy-D-glucose (FDG), which enters the
cell through glucose transporters (GLUTs) and is then phosphorylated
by the hexokinase [9, 10]. FDG has been used to assess impairment of
glucose transport and phosphorylation in human skeletal muscle, in
myocardium and in adipose tissue [11-20]. However, the emission from
18F reflects both 18F-FDG and 18F-FDG-6-phosphate, which leads to
uncertainty as to whether compartmental modelling achieves separate
estimations of glucose transport and phosphorylation. To isolate the
step of transmembrane glucose transport, dynamic PET imaging of 11C-3-O-methyl-D-glucose
(3-OMG) has been proposed [21]. 3-OMG is a glucose analogue which is
also transported into the cell through GLUTs. Unlike FDG, 3-OMG is not
further metabolised, and its transport across the cellular membrane is
therefore bi-directional [22, 23]. The cellular uptake of 3-OMG allows
true glucose transport rates to be determined. 3-OMG has been used in
humans to study regional glucose transport in the brain, the heart
[24, 25] and, more recently, the skeletal muscle [21]. However, the
use of this tracer is limited by the fact that it is labelled with a
very short half-life radioisotope (11C, t1/2=20 min), which
undoubtedly explains why very few studies have been devoted to this
compound and excludes routine clinical use. 14C-labelled 3-OMG has
been used in vivo to identify glucose transport defects in the
skeletal muscle of patients with type 2 diabetes [26-28]. However, 14C
is a -emitter that does not allow in vivo imaging. Moreover, its long
half-life precludes its use in clinical practice.
123I-6-deoxy-6-iodo-D-glucose (6DIG) is a radiolabelled tracer of
glucose transport that was previously described by our laboratory and
proposed as an alternative tracer to assess insulin resistance in vivo
[29]. The biological behaviour of 6DIG is similar to that of 3-OMG
[30]. An in vitro study on adipocytes from diabetic rats and obese
mice showed that 6DIG, like 3-OMG, could be used to determine
alterations in glucose transport [31]. Finally, an in vivo study in
diabetic db/db mice showed that 6DIG was able to identify defects in
glucose transport associated with the presence of type 2 diabetes
[32]. However, the ability of 6DIG to identify the more clinically
relevant pre-diabetic state of insulin resistance has not yet been
evaluated. The hypothesis tested in the present study was that 6DIG
would allow the identification of variations in glucose transport in
such pre-diabetic model. Steady-state, well-controlled euglycaemic and
hyperinsulinaemic conditions were obtained and mathematical modelling
was used to discriminate between non-diabetic, insulin-resistant rats
and control rats. This study sets the stage for the future use of 6DIG
as a non-invasive means for the evaluation of insulin resistance.
Materials and methods
Biological Material
Animals were provided by Iffa Credo (Les Arbresles, France), diets by
UAR (Lyon, France), insulin (Umuline 40) by Novo Nordisk (Paris,
France), and insulin radioimmunoassay kit and 125I by Cis Bio
International (Paris, France).
Synthesis and labelling of 6DIG
6DIG was obtained as previously described [33]. Since the labelled
glucose was stable for at least 1 month as determined by
high-performance liquid chromatography (data not shown), 125I (t1/2=60
days) labelling was used for this study instead of 123I (t1/2=13 h) to
avoid repetitive labelling of the compound. The radiolabelling of 6DIG
was performed by 125I - 127I isotopic exchange according to a
previously described method [32].
Biological Procedure
Animals. Ninety-six male Wistar rats weighing about 50 g (3 weeks old,
just after weaning) were housed (five animals/cage) in an
environmentally controlled room with a 12-h light/dark cycle and free
access to laboratory diet and water. The animals were divided into two
groups: the control group received a standard UAR 210 diet (n=48) and
the fructose-fed group received the UAR 210 "fructose" diet (n=48) in
which fructose composed 56.8% of total carbohydrates [34, 35]. The
rats were numbered and their body weight was monitored weekly for 6-7
weeks.
Protocols. All rats were catheterised in the jugular vein and the
carotid artery as previously described and studies were conducted 24 h
thereafter in the fasting and unrestrained conscious state [34, 35].
For each group of rats, two experiments were performed: a
euglycaemic-hyperinsulinaemic clamp with an insulin infusion rate of 2
mIU.min-1 (40 pmol.min-1.kg-1) and a variable infusion rate of glucose
(20% wt/vol) (n=24), and a “sham-operated” protocol with a simple
infusion of an isotonic saline solution (n=24) (Fig. 1). Arterial
blood samples were obtained at baseline and every 5 min for 60 min
during the isotonic saline control or insulin clamp studies (0-60 min)
for serial determination of glycaemia using a YSI 2300 STAT Plus
glucose analyzer. Glycaemia monitoring allowed adjustment of the
glucose infusion rate (GIR) so that blood glucose concentration
remained at 5 mM for each group. When glycaemia had reached a steady
state, a bolus of 125I-6DIG was injected through the arterial catheter
(15 nmoles in 100 l or 74-111 kBq). Animals were killed by
decapitation at 2, 3, 5, 7, 10, 15 or 20 min post injection (p.i.) (n=3-4
for each time). Immediately prior to euthanasia, 200 l of arterial
blood were collected, quickly centrifuged and frozen for measurement
of plasma insulin concentration. Samples from the blood, heart,
diaphragm, lungs, liver, duodenum, kidneys, abdominal fat, epididymal
fat, quadriceps muscle and brain (cortex) were rapidly obtained. For
reasons of clarity, only data from the blood, heart, diaphragm,
quadriceps muscle, abdominal fat and lungs will only be presented, but
all organs were taken into account for the development of the
mathematical model (see below). The organs were rinsed, weighed, and
the radioactivity was assessed as described below.
Calculations. At high levels of insulin infusion, the rate of glucose
disappearance from the blood (Rd) can be determined by the GIR. Since
previous experiments in our laboratory have shown that in similar
experimental conditions in an identical animal model, hepatic glucose
production was totally inhibited by insulin [36], the GIR obtained in
our study reflected the insulin sensitivity of peripheral tissues. GIR
was measured for each rat at the end of the experiment and expressed
as micromoles of glucose infused per minute and per kilogram (mol.min-1.kg-1).
Glucose and insulin analysis. Baseline glycaemia of each rat
represented the mean of three values obtained during the 15-min basal
state. Results were expressed as mM. Plasma insulin concentration was
determined by radioimmunoassay and was expressed as pmol/l.
Radioactivity counting and expression. Gamma radioactivity was counted
directly in the blood and organs using a gamma-well counter (Cobra II,
Packard). Results were expressed as a percentage of the injected dose
per gram of organ or per millilitre of blood (%ID/g or %ID/ml).
Statistical analysis. Data were presented as mean±SEM. Comparisons
were performed using Student's t test for unpaired values. Kinetics
were compared using repeated measures ANOVA. p values <0.05 were
considered statistically significant.
Mathematical Material
The software SAAM II (Simulation, Analysis And Modeling) was used to
develop a compartmental model and the associated differential
equations to perform parameter estimation and to fit to the data.
Mathematical Procedure
Mathematical model. The model developed to assess 6DIG transport was a
multicompartmental, mamillary model derived from the one used to
define the total body distribution kinetics of FDG [37]. It was used
to fit the behaviour of the tracer following injection in rats in vivo.
The central compartment represented the plasma (q1) in which the
tracer was injected and from which an irreversible loss occurred (k0,1)
[38]. This compartment had bi-directional and linear flux with 11
compartments, which represented the studied organs, q2 to q12.
Radioactivity was measured in these compartments, labelled s1 to s12
respectively (Fig. 2).
Assuming our model is linear and at steady state, then the general
equation is:
where kij is the fractional rate of transfer of the tracer from
compartment j to compartment i (j ≠ i). Measurement error was assumed
to be additive, uncorrelated and zero mean.
Experimental measurements were obtained at precise times (2, 3, 5, 7,
10, 15 and 20 min p.i.) and represented a theoretical output sampling
of the model. For biological systems, the knowledge of the noise
induced by experimental errors is limited. However, it is generally
accepted that the noise is additive and zero mean, that measurement
errors are independent and that the noise follows a Gaussian
distribution [38, 39].
Overall identification of the system. The structural identification
defines the theoretical aspect of model identification; it verifies
the number of acceptable solutions by the equation system describing
the model and the measurements performed [40]. It consists in the
resolution of a non-linear algebraic equation system, which increases
in number of terms and degrees of non-linearity with the model order.
In the present study, the volume of the blood compartment (V) and the
weight of the organs (m2, m3,..., m12) were fixed to obtain a unique
identification of the transfer parameters kij of the model [40, 41].
Numerical identification. Parameters were estimated on the basis of
the assumption that the radioactivity measurements are described by: z(ti)
= y(ti) + e(ti) i = 1..., N, where e represents the measurement error
and N is the number of time measurements (N = 7). Measurement error
was assumed to be additive, independent and zero mean: . The
mathematical model was applied to the experimental data using SAAM II
software and enabled indirect quantification of the physiological
parameters, the fractional transfer coefficients (kij) not being
accessible directly.
Precision of parameters. The results of the model identification were
appreciated by the evaluation of the residual errors (resij), which
were calculated at different time points as the difference between the
measured and the model-predicted values: resij = [s( ,tij) - yi,j],
where s( ,tij) is the model-predicted value, yi,j is the
measured value and is the estimated unknown parameter.
Statistical analysis. Data were reported as values with coefficients
of variation (CV). Comparisons within and between groups were
performed using Student's t test for unpaired data with a significance
level at 5%. To verify the normality of the residual distribution
obtained after adjustment of the model to the data, a
Kolmogorov-Smirnov test was used, based on the comparison of the
distribution function of the normal law N(; ) with the distribution
function obtained for the residues. The hypothesis of normal
distribution, with a significance level of 5%, is accepted if the
value obtained with the test is lower than the critical value (given
in the appropriate table) and rejected if it is not.
Results
Characteristics of control and fructose-fed animals
The results are depicted in Table 1. The “fructose” diet had no effect
on the mean body weight of rats. Fasting glycaemia was not
significantly different in control and fructose-fed rats (4.94±0.05 mM
and 5.27±0.16 mM, respectively, p=NS). Basal plasma insulin
concentration was higher in fructose-fed rats than in control rats
(724±83 pmol/L vs 331±20pmol/L, respectively, p<0.001). Insulin clamp
resulted in a fourfold increase in the steady-state plasma insulin
concentration (p<0.001) in control rats and a twofold increase in this
value in fructose-fed rats (p<0.01). At the end of the insulin clamp,
insulin concentrations in control and fructose-fed rats were not
significantly different, being close to 1,300 pmol/l (Table 1). The
GIR required to obtain euglycaemia (5 mM) was significantly lower in
fructose-fed rats than in control rats (61.4±2.4 mol.min-1.kg-1 vs
101.0±3.4 mol.min-1.kg-1, p<0.001).
Biodistribution of 6DIG
Figures 3 and 4 depict the biodistributions of 6DIG in control and
fructose-fed rats respectively, in basal conditions (panels a) and in
euglycaemic-hyperinsulinaemic conditions (panels b). The results
indicated that circulating 6DIG activity decreased from 2 to 20
minutes following tracer injection. The blood kinetics of the tracer
were similar in the absence or in the presence of insulin in both
experimental groups (Table 2). In the non-insulin-sensitive organs
studied, the evolution of 6DIG activity was comparable to that
observed in the blood and no significant difference was observed
during insulin clamp. Regarding insulin-sensitive organs, in the heart
and the diaphragm of control and fructose-fed rats there was a frank
and significant increase of 6DIG activity at early time points
following perfusion of insulin although the difference was less
dramatic in fructose-fed rats (Figs. 3, 4). This effect of insulin was
also observed in a kinetic point of view (p<0.001 and p<0.01 for the
heart and diaphragm in control rats, respectively; p<0.05 and p=NS for
the heart and diaphragm in fructose-fed rats, respectively) (Table 2).
A particular behaviour was observed in the skeletal muscle, in which
6DIG radioactivity increased with time, from 0.17±0.03 %ID/g to
0.24±0.02 %ID/g in control rats (Fig. 3a) and from 0.15±0.01 %ID/g to
0.28±0.02 %ID/g in fructose-fed rats (Fig. 4a). A significant increase
of 6DIG activity under insulin clamp was observed in skeletal muscle
but in a less extend and at later time points than in the heart and
diaphragm (Figs. 3, 4). The effect of insulin was also observed on the
muscle 6DIG kinetics (p<0.05 in control rats and p=NS in fructose
rats) (Table 2). In the abdominal fat of control and fructose-fed
rats, 6DIG radioactivity was very low, less than 0.1 %ID/g in both
conditions.
Mathematical modelling
Compartmental analysis of 6DIG kinetics. The 6DIG kinetics obtained in
the blood and organs were analysed using the multicompartmental model
shown in Fig. 2. Compartment 1 represented the distribution volume of
the tracer immediately after injection. It was assumed to be equal to
the blood volume, which represents 4 ml/100 g of body weight in the
rat [42, 43]. The elimination of 6DIG from the body was considered as
an irreversible flux from compartment 1 (k0,1), whereas the exchanges
of 6DIG between compartment 1 and the other compartments (q2 to q12)
were represented by the rate constant into (kij) and out of (kji) the
compartment (Table 3). These parameters were estimated after
adjustment of the model to the experimental data. All the model
parameters were estimated with good precision (coefficients of
variation <100 %). Representative examples of the adjustment of the
model to the experimental data for insulin-sensitive organs of control
and fructose-fed rats are shown in Figs. 5 and 6, respectively. Figure
7 presents the adjustment of the model for the plasma of control
animals. It can be concluded from the results that the model correctly
fitted the data and that it was therefore adequate for describing 6DIG
transport kinetics. The evaluation of residual errors for the 6DIG
kinetics in insulin-sensitive organs and plasma of control rats is
represented in Figs. 8 and 9, respectively. The residues were well
distributed around zero, indicating that the model was adequate for
the description of the 6DIG experimental data. Moreover, the average
residues were below 0.2% for insulin-sensitive organ and below 0.6%
for plasma. Their distribution was random and followed a normal law
(data not shown).
Comparison between sham-operated and insulin clamp conditions in
control rats. The results are presented in Table 3. In the presence of
insulin, the irreversible flux (k0,1) was significantly decreased by
28%, from 0.329 (18%) min-1 during the sham-operated protocol to 0.236
(6%) min-1 during insulin clamp (p<0.05). The fractional transfer
coefficients of 6DIG from the blood into insulin-sensitive organs were
significantly increased in the presence of insulin. Specifically, k2,1
(heart), k3,1 (skeletal muscle) and k4,1 (diaphragm) were respectively
increased 5-fold (p<0.01), 3.5-fold (p<0.001) and 2.5-fold (p<0.001)
during the insulin clamp protocol. No significant increase in 6DIG
transport was observed in the adipose tissue in the presence of
insulin. The fractional transfer coefficients out of the organs were
also significantly increased. k1,2 (heart), k1,3 (skeletal muscle) and
k1,4 (diaphragm) were respectively increased 3.7-fold (p<0.05),
3.5-fold (p<0.01) and 2.7-fold (p<0.05) during the insulin clamp
protocol. In non-insulin-sensitive organs, insulin had no significant
effect on 6DIG fractional transfer coefficients.
Comparison between sham-operated and insulin clamp conditions in
fructose-fed rats (Table 3). In the presence of insulin, the
irreversible flux (k0,1) was significantly decreased by 30% in
fructose-fed rats, from 0.214 (6%) min-1 in sham-operated protocol to
0.149 (18%) min-1 during the insulin clamp (p<0.05). In
insulin-sensitive organs, insulin had only a slight effect on the 6DIG
transport into and out of the organs, which was not significant in
most of these organs. As an example, the fractional transfer
coefficients of 6DIG in the heart (k2,1 and k1,2) were not
statistically different in the absence or presence of insulin [k2,1=
0.004 (21%) min-1 in sham-operated condition vs 0.012 (44%) min-1
during insulin clamp, p=NS); k1,2=0.145 (32%) min-1 in sham-operated
condition vs 0.364 (46%) min-1 during insulin clamp, p=NS]. In non
insulin-sensitive organs, no significant difference was observed
between sham-operated and insulin clamp conditions.
Discussion
Insulin resistance is primarily characterised by a defect in glucose
transport following insulin stimulation. This phenomenon has been
observed in numerous pathological disorders such as type 2 diabetes,
cardiovascular disease (syndrome X) and obesity [5, 44]. 6DIG is a
radiolabelled tracer of glucose transport which has a biological
behaviour similar to that of 3-OMG and has been proposed for the
assessment of insulin resistance in vivo by nuclear imaging [30-32].
The ability of 6DIG to identify the more clinically relevant
pre-diabetic state of insulin resistance has not yet been determined.
Accordingly, the present study was performed in an animal model, the
fructose-fed rats [34, 35]. The main finding of this study is that
kinetics of 6DIG allowed the identification of moderately insulin
resistant animals and mathematical modelling made it possible to
obtain numerical parameters. The heart was the organ in which insulin
had the most important effect, with a difference potentially
sufficient to be detected by non-invasive nuclear imaging in vivo and
quantified with an adapted model.
In control and fructose-fed animals, the kinetics of 6DIG were
performed during the steady-state of a sham and an
euglycaemic-hyperinsulinaemic clamp protocol. This last method allowed
us (1) to directly and independently assess insulin resistance through
the measurement of the GIR and (2) to reach steady state conditions
necessary to evaluate the kinetics of 6DIG in the setting of
hyperinsulinaemia and normoglycaemia. Our data showed that insulin
resistance of fructose-fed rats, reflected by a GIR 40% lower than
that of the control rats, was associated with compensatory
hyperinsulinaemia at baseline, a typical feature of the pre-diabetic
state, whereas baseline glycaemia was not significantly different.
During insulin clamp, the plasma insulin concentration increased in
control and fructose-fed animals to reach a comparable value of ~1,300
pmol/l whereas the glycaemia did not change. In both conditions and
both groups of rats, 6DIG radioactivity evolution in most of the
organs was identical to that observed in the blood. These results are
comparable to those obtained in wild type db/+ mice, supporting the
fact that 6DIG, a non-phosphorylable glucose transport tracer, reaches
an equilibrium between the blood and tissues [32].
Given the particular behaviour of 6DIG, which rapidly reaches
equilibrium between the plasma and extra- and intracellular spaces, we
developed a mathematical model to quantify the inward and outward
transfer rates of the tracer in each organ. This mathematical model,
well adapted and applied to the kinetic values, enabled us to quantify
the physiological parameters (k) which cannot be measured directly.
The model chosen for the study was multicompartmental, mamillary and
linear. This model is relatively simple because of the limited number
of points, provided a good fit to the data and led to good estimation
of the model parameters (kij). The central compartment is supposed to
represent the plasma, but it actually represents a pool where very
fast exchanges between plasma and the erythrocytes occur [45]. In our
model, we did not take into account these flows of matter, which are
extremely fast, and we rather focused on flows between plasma and the
intracellular compartments of the 11 organs studied, which have
slightly slower kinetics. It is important to point out that this model
was used with a specific aim, namely to measure fractional amounts of
the tracer entering and leaving the various tissue compartments. The
extracellular compartments are supposed to be included in the
compartment "organ"; thus each compartment body contains both the
extra- and the intracellular spaces. Such a constraint was imposed on
the model for reasons of numerical identifiabilities [41]. Indeed, if
the extracellular space is separated from the intracellular space for
each organ, there is an additional compartment for exchanging matter
(bi-directional flows), resulting in two fractional transfer
coefficients for estimating each organ. Considering the number of
experimental points, it would be numerically impossible for the model
to estimate the 22 parameters present in such a model.
In the Michaëlis kinetic type of transmembrane glucose transport,
fractional transfer coefficients (kij), tracer amount (qi), Michaëlis
constant (Km) and maximal rate (Vm) are defined as follows: (kij)=(Vmj)/(Kmj+qj)
et (kji)=(Vmi)/(Kmi+qi). The linearity of the exchanges between the
plasma compartment and the organ compartments is justified by the use
of small quantities of tracer such that qi<
state. In this case, fractional transfer coefficients are: (kij)=(Vmj)/(Kmj)
and (kji)=(Vmi)/(Kmi). In addition, Km and Vm values under basal
conditions and after insulin exposure had been provided in a study of
6DIG transport on adipocytes [31]. In our study, Vm values were
determined on the basis of the input constant (k2,1) obtained for the
heart and the Km measured on adipocytes. The results obtained are as
follow: basal condition: Vm=12 nmol.min-1.ml-1 for the heart (vs 11
nmol.min-1.ml-1 for adipocytes), and insulin condition: Vm=60 nmol.min-1.ml-1
for the heart (vs 91 nmol.min-1.ml-1 for adipocytes). The maximal rate
of transport was therefore increased sixfold in the presence of
insulin in vivo, and the computed values seemed reasonably in
agreement with the values obtained for the adipocytes in vitro. The
linear model thus provided Vm and Km values of the same order of
magnitude as those found in the literature [31]. The most interesting
feature of the model is its application to a large number of organs in
addition to the blood. It was also the most suitable model for our
study both for the structure and the estimation of parameters. The
coefficients of variation were slightly high because of the restricted
number of animal studied. However, they were sufficient to detect
significant differences between groups.
In control rats, the loss represented by (k0,1) was less important
during the clamp (0.236 min-1 under insulin clamp vs 0.329 min-1 in
the sham-operated protocol, p<0.05), confirming that in such
conditions the tracer was present in greater quantities in the organs.
No significant changes in 6DIG uptake were observed with insulin in
the non-sensitive organs or the adipose tissue. Tracer uptake in
adipose tissue was very low in both conditions. Henry et al. have
shown in vitro that insulin increased 6DIG transport in adipocytes
isolated from both rats and mice and that this stimulation was greater
in rat than in mouse adipocytes, in agreement with their respective
number of GLUT4 transporters [31]. The discrepancy between the in vivo
and in vitro action of insulin on 6DIG activity in the adipose tissue
has also been reported for 2-DG, a tracer of glucose transport and
phosphorylation [46]. The low uptake of 6DIG and 2-DG observed in vivo
could be due to the poor vascularisation of this tissue. Moreover,
James et al. calculated that the increase in white adipose tissue
glucose utilisation during hyperinsulinaemia for a 400-g rat with
15-20% body fat represented at most only 3% of the whole-body GIR
[46]. This and the relatively low level of hyperinsulinaemia are
probably the reasons why we were unable to see any changes in 6DIG
activity in the adipose tissue during the insulin clamp protocol.
In both conditions and for both groups of rats, skeletal muscle
kinetics differed markedly from those observed in other organs, and
the adjustment of the model to the experimental data obtained in this
organ was suboptimal. Indeed, 6DIG uptake increased slightly between 2
and 7 min and then reached a plateau until 20 min. While 6DIG was
quickly eliminated from the other organs, it therefore seemed to be
retained in the skeletal muscle. This retention has also been recently
observed in vivo in human with PET imaging of the glucose transport
tracer 11C-3OMG [21]. This suboptimal result could be explained by the
fact that the multicompartmental model used in the present study is
based on the assumption that all tissue kinetics are similar.
Moreover, Saccomani et al. and Bonadonna et al. had to develop a very
complex model to measure glucose transport in skeletal muscle with
tracers owing to the heterogeneity of this tissue [45, 3]. Skeletal
muscle is quantitatively the most important glucose-utilising tissue,
accounting for 70-80% of glucose utilised during hyperinsulinaemia,
and is the principal site of insulin resistance [48]. Most experiments
using radioactive tracers to study glucose transport stimulated by
insulin in vivo have been performed on human forearm or leg muscles
[3, 11-14, 16, 17, 25-28]. Therefore, further additional studies are
currently underway to fully characterise 6DIG kinetics in this
particular organ.
Our aim was to identify glucose transport variations in vivo with 6DIG
to allow further discrimination between control and insulin-resistant
rats. In our conditions, the heart and the diaphragm were the organs
in which insulin had the most important effect on the model
parameters. Indeed, after 2 min, 6DIG uptake in the heart doubled and
k2,1 was multiplied by 5 whereas k1,2 showed only a threefold
increase, and in the diaphragm, k4,1 was multiplied by 2.5. However,
the size of this last organ precludes it as a target for imaging.
Nevertheless, the differences observed in the heart are theoretically
sufficient to be detected by nuclear imaging in vivo and to be
quantified with a suitable adapted model. In fructose-fed rats,
insulin had only a slight effect on the 6DIG transport into and out of
the organs, which was not significant in most organs, whether they
were insulin sensitive or not.
In conclusion, the present study has shown for the first time that it
is possible to identify variations of glucose transport in vivo in a
pre-diabetic animal model using the glucose transport tracer 6DIG.
However, further studies are needed to determine whether non-invasive
nuclear imaging of 6DIG in the heart will allow the discrimination of
control and insulin-resistant animals. In this setting, the greater
number of experimental time points provided by non-invasive imaging
should allow superior temporal resolution and therefore excellent
numerical estimation of the model parameters.
Acknowledgements
This work was financially supported by the National Institute for
Health and Medical Research (INSERM, France) and by the Headquarters
of Atomic Energy (CEA, France). All experiments were reviewed,
approved and performed under the authority of individuals allowed to
work on living animals by the French government (C. Ghezzi,
authorisation 38-01).
References
1. Zimmet P, Alberti K, Shaw J. Global and societal implications of
the diabetes epidemic. Nature 2001; 414:782-87.
2. Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs
at least 4-7 years before clinical diagnosis. Diabetes Care 1992; 15:815-19.
3. Bonadonna RC, Saccomani MP, Seely L, Zych KS, Ferrannini E, Cobelli
C, et al. Glucose transport in human skeletal muscle. The in vivo
response to insulin. Diabetes 1993; 42:191-98.
4. Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD.
Evidence for defects in the trafficking and translocation of GLUT4
glucose transporters in skeletal muscle as a cause of human insulin
resistance. J Clin Invest 1998; 101:2377-86.
5. Garvey WT, Birnbaum MJ. Cellular insulin action and insulin
resistance. In: Ferrannini E, editors. Bailleiere's Clinical
Endocrinology and Metabolism: Insulin resistance and disease. London:
Bailleiere Tindall; 1994; p. 785-873.
6. Eriksson J, Franssila-Kallunki A, Ekstrand A, Saloranta C, Widen E,
Schalin C, et al. Early metabolic defects in persons at increased risk
for non-insulin-dependent diabetes mellitus. N Engl J Med 1989; 321:337-43.
7. Taylor SI, Accili D, Imai Y. Insulin resistance or insulin
deficiency. Which is the primary cause of NIDDM? Diabetes 1994; 43:735-40.
8. Radziuk J. Insulin sensitivity and its measurement: structural
commonalities among the methods. J Clin End Metab 2000; 85:4426-33.
9. Bessell EM, Foster AB, Westwood JH. The use of
deoxyfluoro-D-glucopyranoses and related compounds in a study of yeast
hexokinase specificity. Biochem J 1972; 128:199-204.
10. Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan C-N, Wolf
AP. Metabolic trapping as a principle of radiopharmaceutical design:
some factors responsible for the biodistribution of [18F]
2-deoxy-2-fluoro-D-glucose. J Nucl Med 1978; 19:1154-61.
11. Voipio-Pulkki LM, Nuutila P, Knuuti MJ, Ruotsalainen U, Haaparanta
M, Teras M, et al. Heart and skeletal muscle glucose disposal in type
2 diabetic patients as determined by positron emission tomography. J
Nucl Med 1993; 34:2064-7.
12. Selberg O, Burchert W, vd Hoff J, Meyer GJ, Hundeshagen H, Radoch
E, et al. Insulin resistance in liver cirrhosis. Positron-emission
Tomography scan analysis of skeletal muscle glucose metabolism. J Clin
Invest 1993; 91:1897-1902.
13. Kelley DE, Mintun MA, Watkins SC, Simoneau JA, Jadali F,
Fredrickson A, et al. The effect of non-insulin-dependent diabetes
mellitus and obesity on glucose transport and phosphorylation in
skeletal muscle. J Clin Invest 1996; 97:2705-13.
14. Paternostro G, Camici PG, Lammerstma AA, Marinho N, Baliga RR,
Kooner JS, et al. Cardiac and skeletal muscle insulin resistance in
patients with coronary heart disease. J Clin Invest 1996; 98:2094-99.
15. Ng CK, Soufer R, McNulty PH. Effect of hyperinsulinemia on
myocardial fluorine-18-FDG uptake. J Nucl Med 1998; 39:379-83.
16. Yokoyama I, Ohtake T, Momomura S, Yonekura K, Kobayakawa N, Aoyagi
T, et al. Insulin action on heart and skeletal muscle FDG uptake in
patients with hypertriglyceridemia. J Nucl Med 1999; 40:1116-21.
17. Williams KV, Price JC, Kelley DE. Interactions of impaired glucose
transport and phosphorylation in skeletal muscle insulin resistance.
Diabetes 2001; 50:2069-79.
18. Virtanen KA, Lönnroth P, Parkkola R, Peltoniemi P, Asola M,
Viljanen T, et al. Glucose uptake and perfusion in subcutaneous and
visceral adipose tissue during insulin stimulation in nonobese and
obese humans. J Clin Endocrinol Metab 2002; 87:3902-10.
19. Williams KV, Bertoldo A, Mattioni B, Price JC, Cobelli C, Kelley
DE. Glucose transport and phosphorylation in skeletal muscle in
obesity: insight a muscle-specific positron emission tomography model.
J Clin Endocrinol Metab 2003; 88:1271-79.
20. Virtanen KA, Iozzo P, Hällsten K, Huupponen R, Parkkola R,
Janatruinen T, et al. Increased fat mass compensates for insulin
resistance in abdominal obesity and type 2 diabetes. Diabetes 2005;
54:2720-26.
21. Bertoldo A, Price J, Mathis C, Mason S, Holt D, Kelley C, et al.
Quantitative Assessment of Glucose Transport in Human Skeletal Muscle:
Dynamic Positron Emission Tomography Imaging of
[O-Methyl-11C]3-O-Methyl-D-Glucose. J Clin Endocrinol Metab 2005;
90:1752-9.
22. Csaky TZ, Wilson JE. The fate of 3-O-14CH3-glucose in the rat.
Biochim Biophys Acta 1956; 22:185-6.
23. Kloster G, Müller-Platz C, Laufer P. 3-[11C]-methyl-D-glucose, a
potential agent for regional cerebral glucose utilization studies:
synthesis, chromatography and tissue distribution in mice. J Labelled
Compd Radiopharm 1981; 18:855-63.
24. Feinendegen LE, Herzog H, Wieler H, Patton DD, Schmid A. Glucose
transport and utilization in the human brain: model using carbon-11
methylglucose and positron emission tomography. J Nucl Med 1986; 27:1867-77.
25. Vyska K, Freundlieb C, Höck A, Becker V, Schmid A, Feinendegen LE,
et al. Analysis of local perfusion rate (LPR) and local glucose
transport rate (LGTR) in brain and heart in man by means of
C-11-methyl-D-glucose (CMG) and dynamic positron emission tomography
(dPET). In: Höfer R, Bergman H, editors. Radioaktive isotope in klinik
und forschung. Vienna: Gasteiner International Symposium Ergmann H;
1982; p. 129-42.
26. Bonadonna RC, Saccomani MP, Cobelli C. In vivo glucose transport
in human skeletal muscle: tools, problems and perspectives. In:
Ferrannini E, editors. Bailleiere's Clinical Endocrinology and
Metabolism: Insulin Resistance and Disease. London: Bailleiere
Tindall; 1993; p. 929-60.
27. Bonadonna RC, Del Prato S, Saccomani MP, Bonora E, Gulli G,
Ferrannini E, et al. Transmembrane glucose transport in skeletal
muscle of patients with Non-Insulin-Dependent Diabetes. J Clin Invest
1993; 92:486-94.
28. Bonadonna RC, Del Prato S, Bonora E, Saccomani MP, Gulli G, Natali
A, et al. Roles of glucose transport and glucose phosphorylation in
muscle insulin resistance of NIDDM. Diabetes 1996; 45:915-25.
29. Bignan G, Ghezzi C, Henry C, Koumanov F, Morin C, Ogier L, et al.
[Iodinated analogues of monosaccharides usable as
radiopharmaceuticals.] French patent N° 95 95214; 1995.
30. Henry C, Koumanov F, Ghezzi C, Morin C, Mathieu J-P, Vidal M, et
al. [123I]-6-deoxy-6-iodo-D-glucose (6DIG), a potential tracer of
glucose transport. Nucl Med Biol 1997; 24:527-34.
31. Henry C, Tanti JF, Grémeaux T, Morin C, Van Obberghen E, Comet M,
et al. Characterization of 6-deoxy-6-iodo-D-glucose. A potential new
tool to assess glucose transport. Nucl Med Biol 1997; 24:99-104.
32. Perret P, Ghezzi C, Mathieu J-P, Morin C, Fagret D. Assessment of
insulin sensitivity in vivo in control and diabetic mice with a
radioactive tracer of glucose transport: [125I]-6-deoxy-6-iodo-D-glucose.
Diabetes Metab Res Rev 2003; 19:306-12.
33. Charronneau E, Mathieu JP, Morin C. Large-scale synthesis and
radiolabelling of 6-deoxy-6-iodo-D-glucose (6DIG). Appl Radioat Isot
1998; 49:1605-7.
34. Faure P, Rossini E, Lafond JL, Richard MJ, Favier A, Halimi S.
Vitamin E improves the free radical defense system potential and
insulin-sensitivity of rats fed high-fructose diets. J Nutr 1997; 127:103-7.
35. Faure P, Rossini E, Wiernsperger N, Richard MJ, Favier A, Halimi
S. An insulin sensitizer improves the free radical defense system
potential and insulin sensitivity in high fructose-fed rats. Diabetes
1999; 48:353-7.
36. Faure P, Lafond JL, Coudray C, Rossini E, Halimi S, Favier A, et
al. Zinc prevents the structural and functional properties of free
radical treated-insulin. Biochim Biophys Acta 1994; 1209:260-4.
37. Hays MT, Segall GM. A mathematical model for the distribution of
fluorodeoxyglucose in humans. J Nucl Med 1998; 40:1358-66.
38. Jacquez JA. Compartmental analysis in biology and medicine. New
York: Elsevier; 1972.
39. Carson ER, Cobelli C, Finkelstein L. The mathematical modelling of
metabolic and endocrine system. New York: Elsevier; 1983.
40. DiStefano III JJ, Cobelli C. Parameter and structural
identifiability concepts and ambiguities: a critical review and
analysis. Am J Physiol 1980; 239:R7-R24.
41. DiStefano III JJ, Jang M, Malone TK, Broutman M. Comprehensive
kinetics of triiodothyronine (T3) production and metabolism in blood
and tissue pools of the rat using optimized blood sampling protocols.
Endocrinology 1982; 110:198-213.
42. Larsson M, Ware J. Effects of isotonic fluid load on plasma water
and extracellular fluid volumes in the rat. Eur Surg Res 1983; 15:262-7.
43. Youn JH, Kim JK, Steil GM. Assessment of extracellular glucose
distribution and glucose transport activity in conscious rats. Am J
Physiol 1995; 268:E712-21.
44. Reaven GM. Role of insulin resistance in human disease. Diabetes
1988; 37:1595-1607.
45. Cobelli C, Toffolo G. A model of glucose kinetics and their
control by insulin, compartmental and noncompartmental approaches.
Math Biosci 1984;72:291-315.
46. James DE, Durleigh KM, Kraegen EW. Time dependence of insulin
action in muscle and adipose tissue in the rat in vivo. An increasing
response in adipose tissue with time. Diabetes 1985; 34:1049-54.
47. Saccomani MP, Bonadonna RC, Bier DM, DeFronzo RA, Cobelli C. A
model to measure insulin effects on glucose transport and
phosphorylation in muscle: a three tracer study. Am J Physiol 1996;
270:E170-85.
48. DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP.
The effect of insulin on the disposal of intravenous glucose. Results
from indirect calorimetry and hepatic and femoral venous
catherization. Diabetes 1981; 30:1000-7.
LEGENDS
Fig. 1. Experimental protocol.
Fig. 2. Selected model for the study of 6DIG transport.
Fig. 3. Biodistribution of 6DIG in sham-operated (a) and clamped (b)
control rats at 2, 3, 5, 7, 10, 15 and 20 minutes post injection.
Fig. 4. Biodistribution of 6DIG in sham-operated (a) and clamped (b)
fructose-fed rats at 2, 3, 5, 7, 10, 15 and 20 minutes post injection.
Fig. 5. Model fit to the experimental data obtained with 6DIG for the
control rat in basal condition (a) and under insulin (b). s2, s3, s4
and s5 respectively represent the kinetics simulated by the model for
the heart, the skeletal muscle, the diaphragm and abdominal fat. Unit
for the y-axis: % injected dose/gram of organ.
Fig. 6. Model fit to the experimental data obtained with 6DIG for the
fructose-fed rat in basal condition (a) and under insulin (b). s2, s3,
s4 and s5 respectively represent the kinetics simulated by the model
for the heart, the skeletal muscle, the diaphragm and abdominal fat.
Unit for the y-axis: % injected dose/gram of organ.
Fig. 7. Model fit to the experimental data obtained with 6DIG for the
plasma compartment for the control rats in the basal condition (a) and
under insulin (b). Unit for the y-axis: % injected dose/millilitre of
blood.
Fig. 8. Residues of the model adjustment to the experimental data
obtained with 6DIG for the control rats in the basal condition (a) and
under insulin (b). s2-res: heart, s3-res: skeletal muscle, s4-res:
diaphragm, s5-res: abdominal fat.
Fig. 9. Residues of the model adjustment to the experimental data
obtained with 6DIG for the plasma compartment for the control rats in
the basal condition (a) and under insulin (b).
Table 1. Characteristics of control and fructose-fed rats.
Control rats
============
Fructose-fed rats
=================
Weight (g)
299.2 ± 1.8
306.5 ± 3.8
Fasting glycaemia (mM)
4.94 ± 0.05
5.27 ± 0.16
Experimental condition
sham insulin
sham insulin
Plasma insulin (pmol/l)
331 ± 20 1369 ± 91*
724 ± 83** 1286 ± 96*
GIR (mol.min-1.kg-1)
- 101.0 ± 3.4
- 61.4 ± 2.4**
*p<0.01 vs sham-operated, **p<0.001 vs control.
Table 2. Comparison of 6DIG kinetics in sham-operated animals vs
insulin clamp animals of the control or fructose-fed group.
Heart
Diaphragm
Muscle
Abd. fat
Lungs
Blood
Control
p<0.001
p<0.01
p<0.05
NS
NS
NS
Fructose-fed
p<0.05
NS
NS
p<0.05
NS
NS
Table 3. Fractional transfer coefficients (kij) obtained with the
mathematical model.
Control rats
Fructose-fed rats
(kij) (min-1)
Sham
Insulin
Sham
Insulin
(k0,1) blood
0.329 (18)
0.236 (6) *
0.214 (6)
0.149 (18) *
(k2,1) heart
0.003 (13)
0.015 (31) **
0.004 (21)
0.012 (44)
(k1,2) heart
0.102 (27)
0.383 (29) *
0.145 (32)
0.364 (46)
(k3,1) muscle
0.244 (21)
0.855 (21) ***
1.578 (28)
0.918 (11)
(k1,3) muscle
0.068 (46)
0.239 (21) **
0.478 (23)
0.188 (20) *
(k4,1) diaphr.
0.002 (22)
0.005 (16) ***
0.002 (14)
0.002 (33)
(k1,4) diaphr.
0.079 (42)
0.217 (17) *
0.128 (16)
0.097 (55)
(k5,1) abd. fat
0.003 (15)
0.004 (19)
0.003 (21)
0.012 (132)
(k1,5) abd. fat
0.177 (22)
0.287 (18)
0.258 (24)
1.335 (124)
(k10,1) lungs
0.009 (29)
0.014 (18)
0.016 (18)
0.012 (21)
(k1,10) lungs
0.173 (36)
0.255 (18)
0.280 (18)
0.202 (23)
Values between parentheses represent the precision of estimated
parameters ( ) expressed as coefficient of variation (in
percentage), CV: 100.
Comparison sham vs insulin: *p<0.05, **p<0.01 and ***p<0.001.
 FORMULÁRIO DE CADASTRAMENTO DE BOLSISTAS DA UNIVERSIDADE ABERTA
FORMULÁRIO DE CADASTRAMENTO DE BOLSISTAS DA UNIVERSIDADE ABERTA SP002B SOLLICITUD D’ANÀLISI ESPECTROMETRIA DE MASSESEXTERN SERVEI DE PROTEOMICA
SP002B SOLLICITUD D’ANÀLISI ESPECTROMETRIA DE MASSESEXTERN SERVEI DE PROTEOMICA FEDERATIA ROMANA DE LUPTE ROMANIAN WRESTLING FEDERATION FÉDÉRATION
FEDERATIA ROMANA DE LUPTE ROMANIAN WRESTLING FEDERATION FÉDÉRATION MANUAL DE PROCEDIMIENTOS CONTENIDO CONTENIDO 2 CATÁLOGO DE
MANUAL DE PROCEDIMIENTOS CONTENIDO CONTENIDO 2 CATÁLOGO DE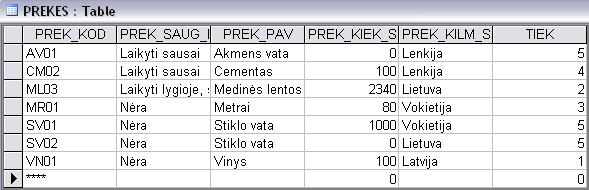 LENTELĖ „PREKĖS“ PREKĖS KODAS 1 ŠIAM LAUKUI PARENKAMAS
LENTELĖ „PREKĖS“ PREKĖS KODAS 1 ŠIAM LAUKUI PARENKAMAS 20151125 ANMÄLAN TILL ÄNGSDALS SKOLOR BUNKEFLOSTRANDS KLAGSHAMNS OCH LIMHAMNS
20151125 ANMÄLAN TILL ÄNGSDALS SKOLOR BUNKEFLOSTRANDS KLAGSHAMNS OCH LIMHAMNS ŠKOLA I ZŠ T G MASARYKA JEŘÁBKOVA 690 399
ŠKOLA I ZŠ T G MASARYKA JEŘÁBKOVA 690 399