experiment 5.4.3f[2] – preparation of methyl 3-nitrobenzoate in two steps !! wear eye protection correctly throughout the experiment !
Experiment 5.4.3f[2] – Preparation of methyl 3-nitrobenzoate in two
steps
!! Wear eye protection correctly throughout the experiment
!! Methanol is toxic by skin absorption
!! Methanol, ethanol, hydrocarbon solvent, hexane and ethoxyethane are
highly flammable
!! No naked flames during chromatography work
!! Do not look directly into the ultra-violet light
In this experiment you will first make some methyl benzoate and then
convert this into methyl 3-nitrobenzoate. You will check the purity of
your product using thin layer chromatography and melting point
determination.
Method
Part 1 - Preparation of methyl benzoate
Add 8 g of benzoic acid to a 50 cm3 pear shaped flask taking care to
keep the flask neck clean. Add 15 cm3 of methanol (CARE!) and 2 cm3 of
concentrated sulfuric acid (CARE!). Set the apparatus up for reflux
and heat for around 45 minutes.
Cool the flask to room temperature and add the contents to a
separating funnel containing 30 cm3 of cold water. Use 15 cm3 of
hydrocarbon solvent (CARE!) to rinse out the flask and add the
washings to the separating funnel. Stopper the separating funnel and
shake it well to mix the contents, making sure that you regularly
release any pressure that builds up. Allow the contents to settle.
Remove the stopper, run off the lower aqueous layer into a conical
flask and discard it.
The product will now be dissolved in the hydrocarbon solvent in the
separating funnel. Add 15 cm3 of distilled water and repeat the
shaking process, again running off and discarding the lower aqueous
layer. Finally, repeat this process using 15 cm3 of 0.5 M sodium
carbonate solution. Take particular care to release any pressure
build-up as carbon dioxide is produced as the sodium carbonate reacts
with, and removes, any acid impurities.
Run the hydrocarbon solvent solution into a conical flask and dry it
by adding one or two spatula measures of anhydrous sodium sulfate. You
should be left with a clear liquid. Filter off the sodium sulfate
solid, collecting the liquid hydrocarbon solution in a clean, dry 50
cm3 pear shaped flask.
Set up the apparatus for distillation and heat the pear shaped flask
to drive off the hydrocarbon solvent. When the temperature reaches 190
ºC change the collection beaker for a fresh, clean, weighed one and
collect the methyl benzoate product boiling above this temperature.
Stop heating when there is little liquid left in the flask being
heated, otherwise it will char. Weigh the beaker and product,
calculate the yield and hence the percentage yield for your reaction.
Part 2 - The nitration of methyl benzoate
Use a measuring cylinder to measure out 9 cm3 of concentrated sulfuric
acid (CARE!) into a 100 cm3 conical flask. Cool the flask below 10 ºC
in an ice bath. Add 4 cm3 of your methyl benzoate liquid whilst
swirling the flask. Meanwhile prepare a mixture of 3 cm3 of
concentrated nitric acid (CARE!) and 3 cm3 of concentrated sulfuric
acid (CARE!) in another conical flask and chill this in the ice bath.
Whilst swirling the methyl benzoate flask, use a dropping pipette to
add the acid mixture (CARE!) dropwise to the methyl benzoate. Keep
chilling the flask in the ice bath, and add the acid at a rate so that
the temperature stays between 5 ºC and 15 ºC. When all the acid has
been added, remove the flask from the ice bath and allow it to stand
for ten minutes. Pour the mixture over 40 g of crushed ice in a 100 cm3
beaker. Stir the mixture and the solid product should precipitate.
When the ice has all melted, collect you solid product by suction
filtration washing it with a few small quantities of cold water.
Replace the Buchner flask for a clean, dry one and give the crystals a
further washing with two 5 cm3 portions of ice-cold ethanol. Don’t
throw this solution away – we shall be using it for the thin layer
chromatography.
Transfer your crude crystals to a 100 cm3 flask and add 15 cm3 of
ethanol. Bring a 400 cm3 beaker half-filled with water to boiling.
Turn the heat off and place your conical flask in it to heat up the
ethanol. When all of the solid has dissolved, remove the flask from
the hot water and place it in an ice bath to recystallise the product.
Recover the methyl 3-nitrobenzoate (it should be a pale yellow solid)
by suction filtration. Let the filtration run for a few minutes in
order to dry your product. Weigh the crystals and work out the overall
percentage yield for the two step process.
Part 3 - Checking purity
Your teacher will demonstrate the melting point apparatus available.
Measure the melting point of your crystals. The melting point of
methyl 3-nitrobenzoate is 78 ºC.
For the chromatography, first evaporate down the ethanol washings that
you kept from part 2. This is done on a water bath and should give you
a final volume of around 1 cm3.
On a thin layer chromatography plate (this will probably be a small
sheet of plastic with a silica coating) mark on a start line about 1
cm from the base using a pencil. Use a fine dropping tube to place a
small spot of the liquid on the baseline towards the left (we need to
put another spot on the baseline). This is the crude product and
should include some methyl 2-nitrobenzoate impurity.
Prepare a solution of your recrystallised product in ethanol. A small
spatula measure in a small volume of ethanol will be sufficient. Place
a spot of this solution on the baseline towards the right.
!! Ensure there are no naked flames in the lab before obtaining the
chromatography solvent. Ethoxyethane is extremely flammable and the
vapour can ignites at large distances from an ignition source.
When the tlc plate has dried, place it in a beaker with about 5 mm
depth of chromatography solvent. Cover the beaker with cling film or a
tile to maintain a solvent atmosphere. Allow the solvent to rise up
the plate until it is one or two centimetres from the top. Remove the
plate from the solvent bath and mark on the solvent front. To view the
chromatogram you will have to look at it under ultraviolet light.
!! Do not look directly into the light.
From your chromatogram, is there any sign that your recrystallisation
has worked?
Student Instruction Sheet Expt 5.4.3f[2] © Practical Chemistry 2009 –
may be copied by licence holders Page 3
 KEY STAGE 1 READING RECORD NAME COLLINS (LILAC
KEY STAGE 1 READING RECORD NAME COLLINS (LILAC  ZAŁĄCZNIK NR 1 DO REGULAMINU UDZIELANIA DOTACJI CELOWEJ NA
ZAŁĄCZNIK NR 1 DO REGULAMINU UDZIELANIA DOTACJI CELOWEJ NA NCSX ENGINEERING DESIGN DOCUMENT DESIGN DESCRIPTION CRYOSTAT AND BASE
NCSX ENGINEERING DESIGN DOCUMENT DESIGN DESCRIPTION CRYOSTAT AND BASE UNIVERSITÉ DE PAU ET DES PAYS DE L’ADOUR COLLÈGE
UNIVERSITÉ DE PAU ET DES PAYS DE L’ADOUR COLLÈGE 2018 CANADIAN MIXED DOUBLES CURLING TRIALS JANUARY 2 –
2018 CANADIAN MIXED DOUBLES CURLING TRIALS JANUARY 2 –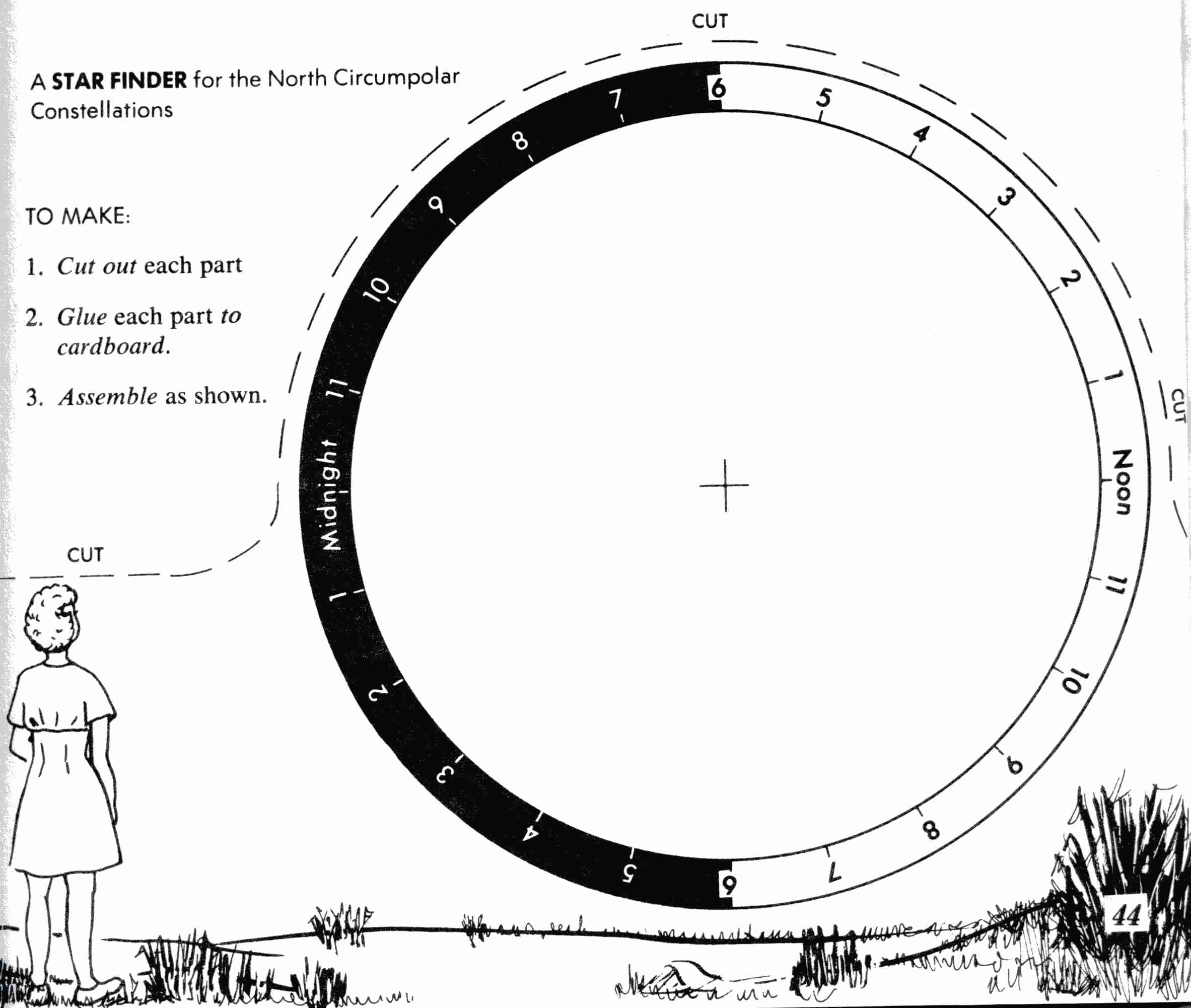 STAR FINDER ACTIVITY CREDIT THE GRAPHICS AND TEXT OF
STAR FINDER ACTIVITY CREDIT THE GRAPHICS AND TEXT OF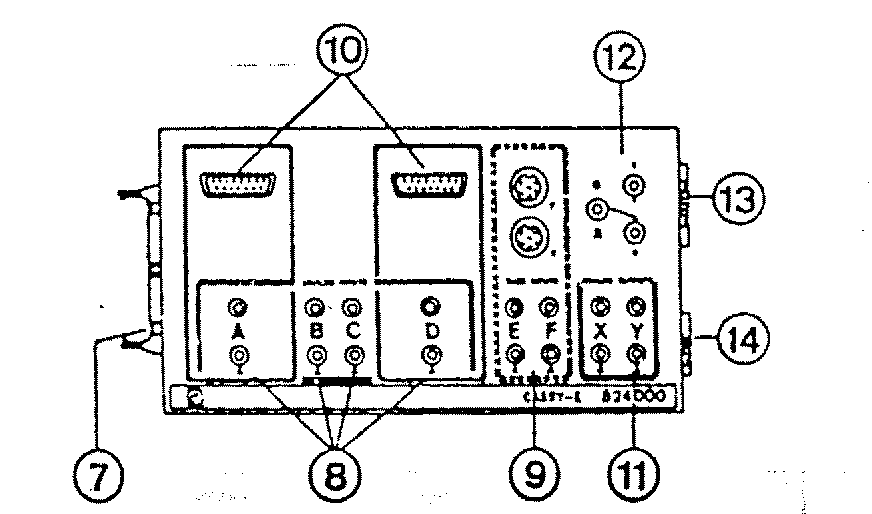 INTERFACE CASSY1 INTRODUCCIÓN EL APARATO QUE DESCRIBIREMOS
INTERFACE CASSY1 INTRODUCCIÓN EL APARATO QUE DESCRIBIREMOS